The Methylome in Female Adolescent Conduct Disorder: Neural Pathomechanisms and Environmental Risk Factors
Post-hoc Analyses
AG Chiocchetti
29 Dezember 2020
Last updated: 2021-09-24
Checks: 7 0
Knit directory: femNATCD_MethSeq/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20210128) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 56bb68f. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/.Rhistory
Ignored: code/.Rhistory
Ignored: data/Epicounts.csv
Ignored: data/Epimeta.csv
Ignored: data/Epitpm.csv
Ignored: data/KangUnivers.txt
Ignored: data/Kang_DataPreprocessing.RData
Ignored: data/Kang_dataset_genesMod_version2.txt
Ignored: data/PatMeta.csv
Ignored: data/ProcessedData.RData
Ignored: data/RTrawdata/
Ignored: data/SNPCommonFilt.csv
Ignored: data/femNAT_PC20.txt
Ignored: output/BrainMod_Enrichemnt.pdf
Ignored: output/Brain_Module_Heatmap.pdf
Ignored: output/DMR_Results.csv
Ignored: output/GOres.xlsx
Ignored: output/LME_GOplot.pdf
Ignored: output/LME_Results.csv
Ignored: output/LME_Results_Sig.csv
Ignored: output/LME_tophit.svg
Ignored: output/ProcessedData.RData
Ignored: output/RNAvsMETplots.pdf
Ignored: output/Regions_GOplot.pdf
Ignored: output/ResultsgroupComp.txt
Ignored: output/SEM_summary_groupEpi_M15.txt
Ignored: output/SEM_summary_groupEpi_M2.txt
Ignored: output/SEM_summary_groupEpi_M_all.txt
Ignored: output/SEM_summary_groupEpi_TopHit.txt
Ignored: output/SEM_summary_groupEpi_all.txt
Ignored: output/SEMplot_Epi_M15.html
Ignored: output/SEMplot_Epi_M15.png
Ignored: output/SEMplot_Epi_M15_files/
Ignored: output/SEMplot_Epi_M2.html
Ignored: output/SEMplot_Epi_M2.png
Ignored: output/SEMplot_Epi_M2_files/
Ignored: output/SEMplot_Epi_M_all.html
Ignored: output/SEMplot_Epi_M_all.png
Ignored: output/SEMplot_Epi_M_all_files/
Ignored: output/SEMplot_Epi_TopHit.html
Ignored: output/SEMplot_Epi_TopHit.png
Ignored: output/SEMplot_Epi_TopHit_files/
Ignored: output/SEMplot_Epi_all.html
Ignored: output/SEMplot_Epi_all.png
Ignored: output/SEMplot_Epi_all_files/
Ignored: output/barplots.pdf
Ignored: output/circos_DMR_tags.svg
Ignored: output/circos_LME_tags.svg
Ignored: output/clinFact.RData
Ignored: output/dds_filt_analyzed.RData
Ignored: output/designh0.RData
Ignored: output/designh1.RData
Ignored: output/envFact.RData
Ignored: output/functional_Enrichemnt.pdf
Ignored: output/gostres.pdf
Ignored: output/modelFact.RData
Ignored: output/resdmr.RData
Ignored: output/resultsdmr_table.RData
Ignored: output/table1_filtered.Rmd
Ignored: output/table1_filtered.docx
Ignored: output/table1_unfiltered.Rmd
Ignored: output/table1_unfiltered.docx
Ignored: setup_built.R
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/03_01_Posthoc_analyses_Gene_Enrichment.Rmd) and HTML (docs/03_01_Posthoc_analyses_Gene_Enrichment.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 56bb68f | achiocch | 2021-09-24 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| Rmd | 43333cc | achiocch | 2021-09-24 | adds new build |
| html | 43333cc | achiocch | 2021-09-24 | adds new build |
| Rmd | dee7132 | achiocch | 2021-09-17 | adds new build |
| html | dee7132 | achiocch | 2021-09-17 | adds new build |
| Rmd | e3b7fcf | achiocch | 2021-09-17 | adds new build |
| html | e3b7fcf | achiocch | 2021-09-17 | adds new build |
| html | b497cc9 | achiocch | 2021-09-17 | Build site. |
| Rmd | 2047ac2 | achiocch | 2021-09-17 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| Rmd | fd80c2d | achiocch | 2021-09-16 | adds SEM improvments |
| html | fd80c2d | achiocch | 2021-09-16 | adds SEM improvments |
| html | ccbc9e4 | achiocch | 2021-08-10 | Build site. |
| Rmd | 723a1aa | achiocch | 2021-08-09 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 70cd649 | achiocch | 2021-08-06 | Build site. |
| Rmd | 611ca24 | achiocch | 2021-08-06 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 2a53a87 | achiocch | 2021-08-06 | Build site. |
| Rmd | e4425b8 | achiocch | 2021-08-06 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | e3a9ae3 | achiocch | 2021-08-04 | Build site. |
| Rmd | 3979990 | achiocch | 2021-08-04 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | f6bbdc0 | achiocch | 2021-08-04 | Build site. |
| Rmd | 1a30b73 | achiocch | 2021-08-04 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 1a30b73 | achiocch | 2021-08-04 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 710904a | achiocch | 2021-08-03 | Build site. |
| Rmd | 16112c3 | achiocch | 2021-08-03 | wflow_publish(c(“analysis/", "code/”, “docs/”)) |
| html | 16112c3 | achiocch | 2021-08-03 | wflow_publish(c(“analysis/", "code/”, “docs/”)) |
| html | cde8384 | achiocch | 2021-08-03 | Build site. |
| Rmd | d3629d5 | achiocch | 2021-08-03 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | d3629d5 | achiocch | 2021-08-03 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | d761be4 | achiocch | 2021-07-31 | Build site. |
| Rmd | b452d2f | achiocch | 2021-07-30 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | b452d2f | achiocch | 2021-07-30 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| Rmd | 1a9f36f | achiocch | 2021-07-30 | reviewed analysis |
| html | 2734c4e | achiocch | 2021-05-08 | Build site. |
| html | a847823 | achiocch | 2021-05-08 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 9cc52f7 | achiocch | 2021-05-08 | Build site. |
| html | 158d0b4 | achiocch | 2021-05-08 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 0f262d1 | achiocch | 2021-05-07 | Build site. |
| html | 5167b90 | achiocch | 2021-05-07 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 05aac7f | achiocch | 2021-04-23 | Build site. |
| Rmd | 5f070a5 | achiocch | 2021-04-23 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | f5c5265 | achiocch | 2021-04-19 | Build site. |
| Rmd | dc9e069 | achiocch | 2021-04-19 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | dc9e069 | achiocch | 2021-04-19 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 17f1eec | achiocch | 2021-04-10 | Build site. |
| Rmd | 4dee231 | achiocch | 2021-04-10 | wflow_publish(c(“analysis/", "code/”, "docs/*")) |
| html | 91de221 | achiocch | 2021-04-05 | Build site. |
| Rmd | b6c6b33 | achiocch | 2021-04-05 | updated GO function, and model def |
| html | 4ea1bba | achiocch | 2021-02-25 | Build site. |
| Rmd | 6c21638 | achiocch | 2021-02-25 | wflow_publish(c(“analysis/", "code/”, "docs/*"), update = F) |
| html | 6c21638 | achiocch | 2021-02-25 | wflow_publish(c(“analysis/", "code/”, "docs/*"), update = F) |
Home = getwd()Sensitivity Analyses
Sensitivity EWAS
including SES
collector=data.frame(originalP=results_Deseq$pvalue,
originall2FC=results_Deseq$log2FoldChange)
rownames(collector)=paste0("Epi", 1:nrow(collector))
parm="EduPar"
workingcopy = dds_filt
workingcopy=workingcopy[,as.vector(!is.na(colData(dds_filt)[parm]))]
modelpar=as.character(design(dds_filt))[2]
tmpmod=gsub("0", paste0("~ 0 +",parm), modelpar)
tmpmod=gsub("int_dis \\+", "", tmpmod)
modelpar=as.formula(tmpmod)
design(workingcopy) = modelpar
workingcopy = DESeq(workingcopy)using pre-existing size factorsestimating dispersionsfound already estimated dispersions, replacing thesegene-wise dispersion estimatesmean-dispersion relationshipfinal dispersion estimatesfitting model and testingparmres=results(workingcopy)
collector[,paste0(parm,"P")] = parmres$pvalue
collector[,paste0(parm,"l2FC")] = parmres$log2FoldChange
idx=collector$originalP<=thresholdp
idx=collector[,paste0(parm,"P")]<=thresholdp
table(collector$originalP<=thresholdp, collector[paste0(parm,"P")]<=thresholdp) %>% fisher.test()
Fisher's Exact Test for Count Data
data: .
p-value < 2.2e-16
alternative hypothesis: true odds ratio is not equal to 1
95 percent confidence interval:
47.31585 57.91749
sample estimates:
odds ratio
52.34226 cor.test(collector$originall2FC[idx],collector[,paste0(parm,"l2FC")][idx],
method = "spearman")
Spearman's rank correlation rho
data: collector$originall2FC[idx] and collector[, paste0(parm, "l2FC")][idx]
S = 199820136, p-value < 2.2e-16
alternative hypothesis: true rho is not equal to 0
sample estimates:
rho
0.9275243 qqplot(y=-log10(collector[,paste0(parm,"P")]),
x = -log(runif(nrow(collector))), xlim=c(0,12),ylim=c(0,12),
col="gray", ylab="", xlab="")
par(new=T)
qqplot(y=-log10(collector$originalP),
x = -log(runif(nrow(collector))),xlim=c(0,12),ylim=c(0,12),
xlab="expected",ylab="observed")
abline(0,1,col="red")
legend("topleft", pch=1, col=c("black", "gray"), legend=c("original", parm))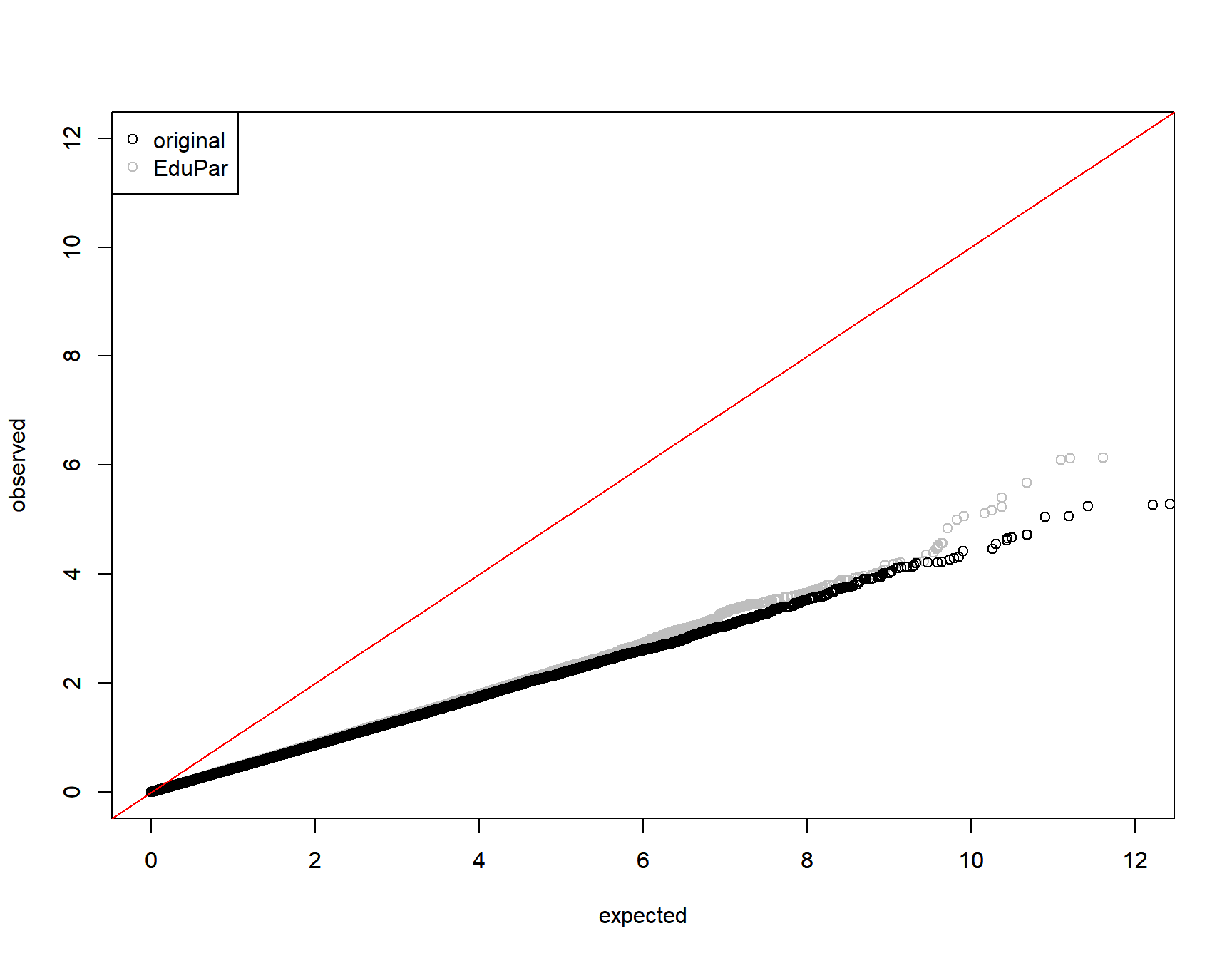
plot(collector$originall2FC[idx],collector[,paste0(parm,"l2FC")][idx], pch=16,
main="log 2 foldchange", ylab=parm, xlab="original")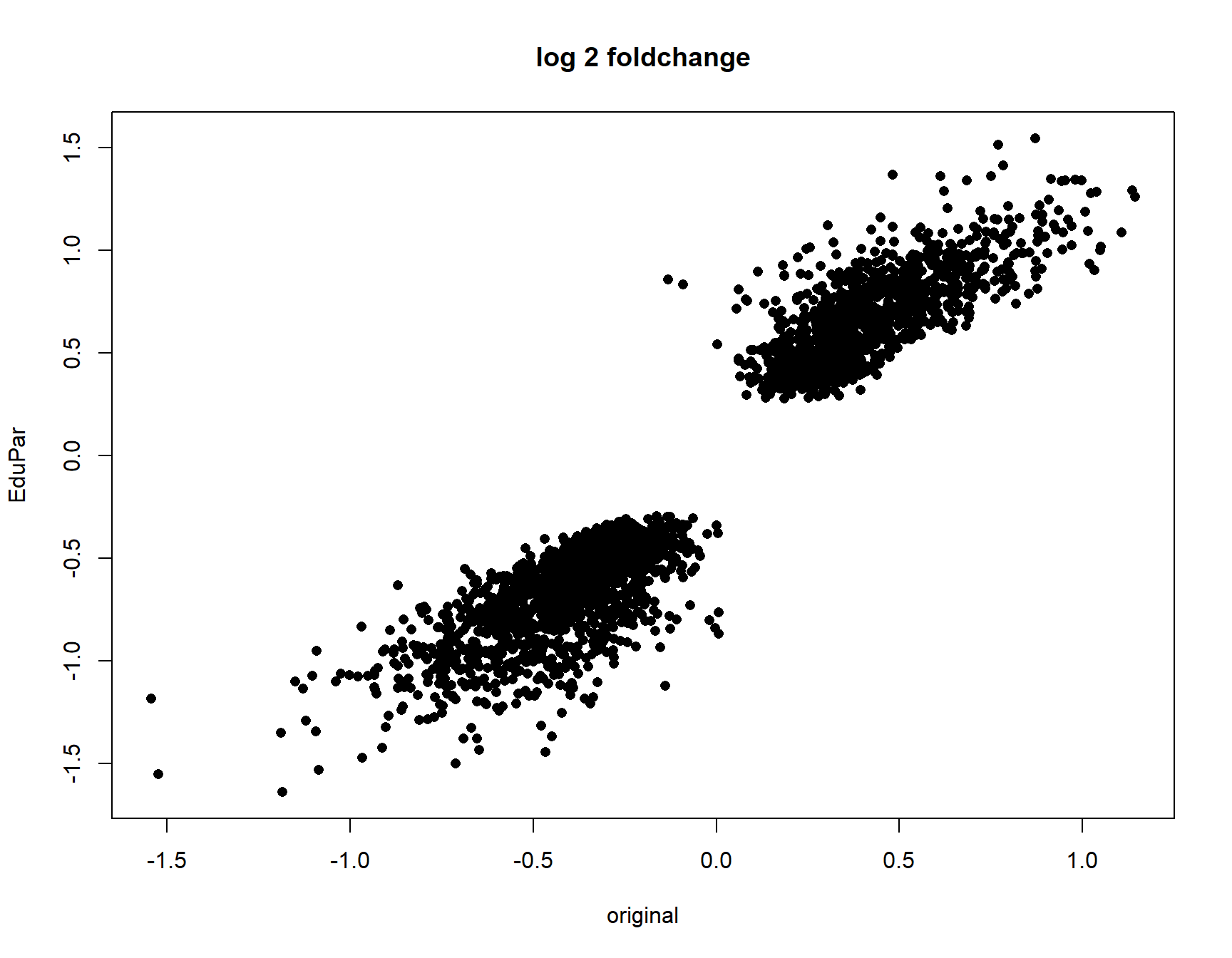
excluding int_dis
### excluding int_dist
modelpar=as.character(design(dds_filt))[2]
modelpar=as.formula(paste("~",gsub("int_dis +", "", modelpar)))
design(workingcopy) = modelpar
workingcopy = DESeq(workingcopy)using pre-existing size factorsestimating dispersionsfound already estimated dispersions, replacing thesegene-wise dispersion estimatesmean-dispersion relationshipfinal dispersion estimatesfitting model and testingparmres=results(workingcopy)
parm="wo.int.dis"
collector[,paste0(parm,"P")] = parmres$pvalue
collector[,paste0(parm,"l2FC")] = parmres$log2FoldChange
idx=collector$originalP<=thresholdp
idx=collector[,paste0(parm,"P")]<=thresholdp
table(collector$originalP<=thresholdp, collector[paste0(parm,"P")]<=thresholdp) %>% fisher.test()
Fisher's Exact Test for Count Data
data: .
p-value < 2.2e-16
alternative hypothesis: true odds ratio is not equal to 1
95 percent confidence interval:
78.91108 96.18862
sample estimates:
odds ratio
87.22894 cor.test(collector$originall2FC[idx],collector[,paste0(parm,"l2FC")][idx],
method = "spearman")
Spearman's rank correlation rho
data: collector$originall2FC[idx] and collector[, paste0(parm, "l2FC")][idx]
S = 122592852, p-value < 2.2e-16
alternative hypothesis: true rho is not equal to 0
sample estimates:
rho
0.9464573 qqplot(y=-log10(collector[,paste0(parm,"P")]),
x = -log(runif(nrow(collector))), xlim=c(0,12),ylim=c(0,12),
col="gray", ylab="", xlab="")
par(new=T)
qqplot(y=-log10(collector$originalP),
x = -log(runif(nrow(collector))),xlim=c(0,12),ylim=c(0,12),
xlab="expected",ylab="observed")
abline(0,1,col="red")
legend("topleft", pch=1, col=c("black", "gray"), legend=c("original", parm))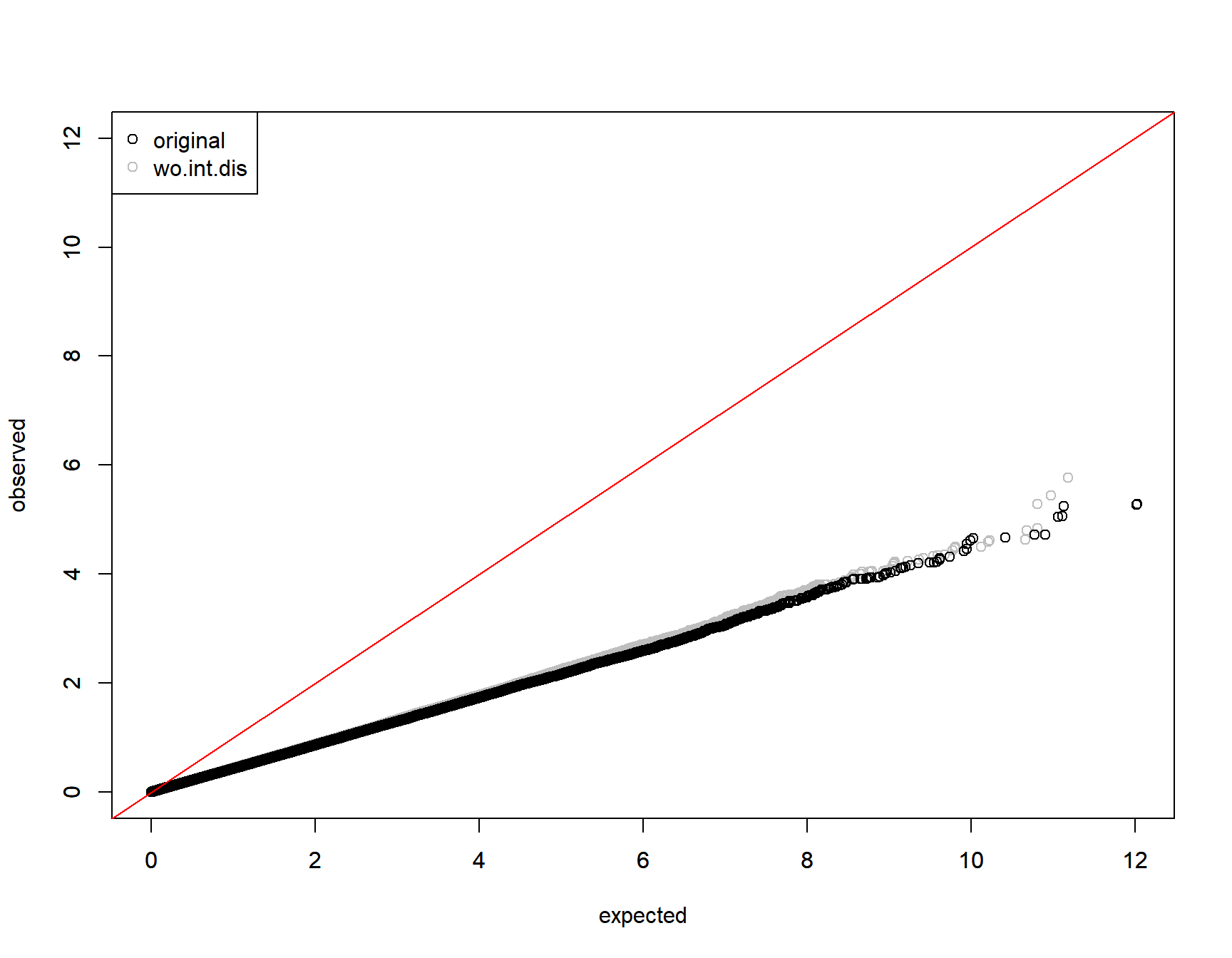
plot(collector$originall2FC[idx],collector[,paste0(parm,"l2FC")][idx], pch=16,
main="log 2 foldchange", ylab=parm, xlab="original")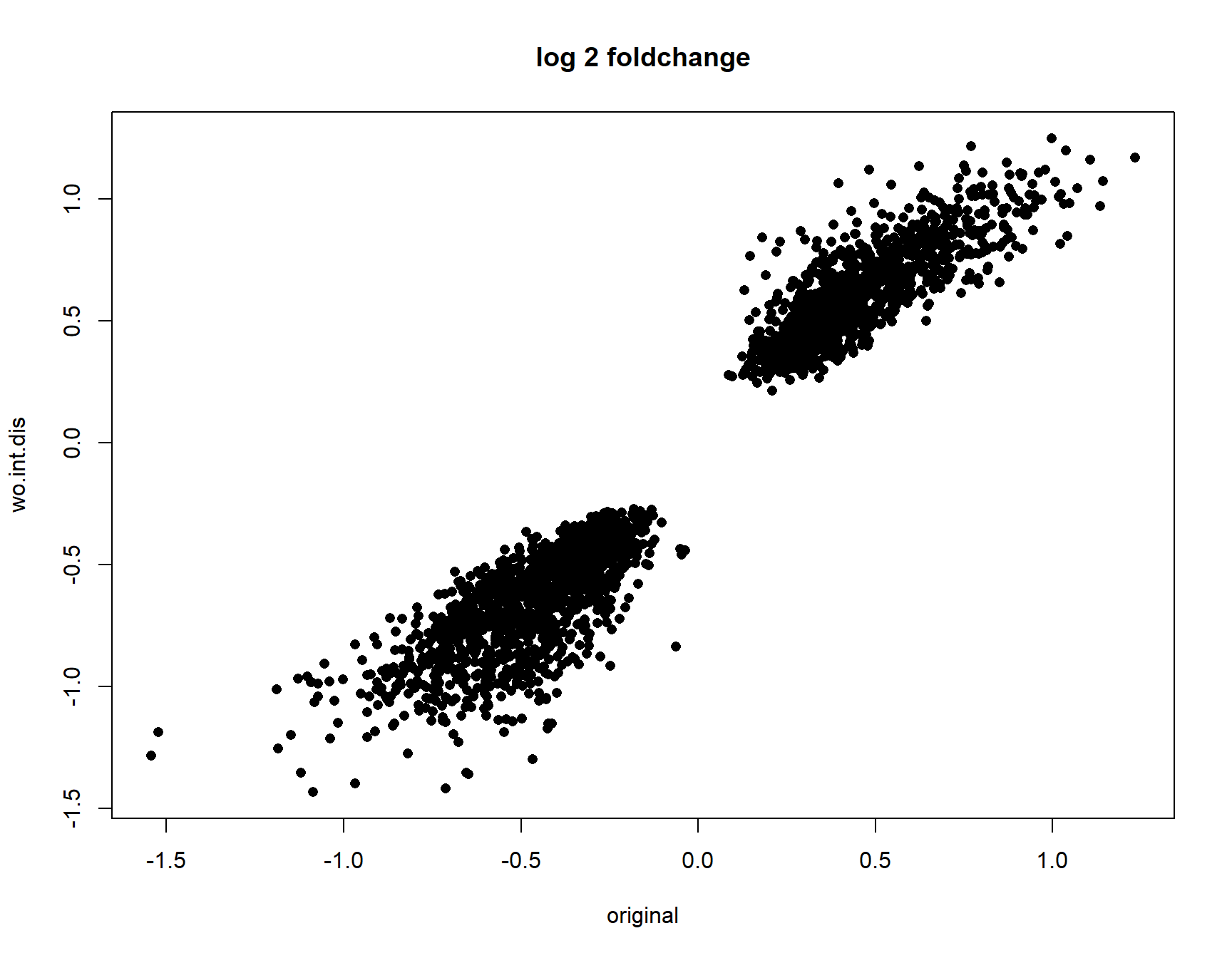
Sensitivity main hit
For the most significant tag of interest (5’ of the SLITRK5 gene), we tested if the group effect is stable if correcting for Ethnicity (PC1-PC4) or CD associated environmental risk factors.
tophit=which.min(results_Deseq$padj)
methdata=log2_cpm[tophit,]
Probdat=as.data.frame(colData(dds_filt))
Probdat$topHit=methdata[rownames(Probdat)]
model0=as.character(design(dds_filt))[2]
model0=as.formula(gsub("0 +", "topHit ~ 0 + ", model0))
lmres=lm(model0, data=Probdat)
lmrescoeff = as.data.frame(coefficients(summary(lmres)))
totestpar=c("site","PC_1", "PC_2", "PC_3", "PC_4", envFact)
ressens=data.frame(matrix(nrow = length(totestpar)+1, ncol=c(3)))
colnames(ressens) = c("beta", "se", "p.value")
rownames(ressens) = c("original", totestpar)
ressens["original",] = lmrescoeff["groupCD", c("Estimate", "Std. Error", "Pr(>|t|)")]
for( parm in totestpar){
modelpar=as.character(design(dds_filt))[2]
modelpar=as.formula(gsub("0", paste0("topHit ~ 0 +",parm), modelpar))
lmres=lm(modelpar, data=Probdat)
lmrescoeff = as.data.frame(coefficients(summary(lmres)))
ressens[parm,] = lmrescoeff["groupCD", c("Estimate", "Std. Error", "Pr(>|t|)")]
}
modelpar=as.character(design(dds_filt))[2]
modelpar=as.formula(gsub("int_dis +", "", gsub("0", "topHit ~ 0", modelpar)))
lmres=lm(modelpar, data=Probdat)
lmrescoeff = as.data.frame(coefficients(summary(lmres)))
ressens["w/o_int_dis",] = lmrescoeff["groupCD", c("Estimate", "Std. Error", "Pr(>|t|)")]
a = barplot(height = ressens$beta,
ylim=rev(range(c(0,ressens$beta-ressens$se)))*1.3,
names.arg = rownames(ressens), col=Set3, border = NA, las=3,
ylab="beta[se]", main="Effect sensitvity analysis")
arrows(a,ressens$beta, a, ressens$beta+ressens$se, angle = 90, length = 0.1)
arrows(a,ressens$beta, a, ressens$beta-ressens$se, angle = 90, length = 0.1)
text(a, min(ressens$beta-ressens$se)*1.15,
formatC(ressens$p.value), cex=0.6, srt=90)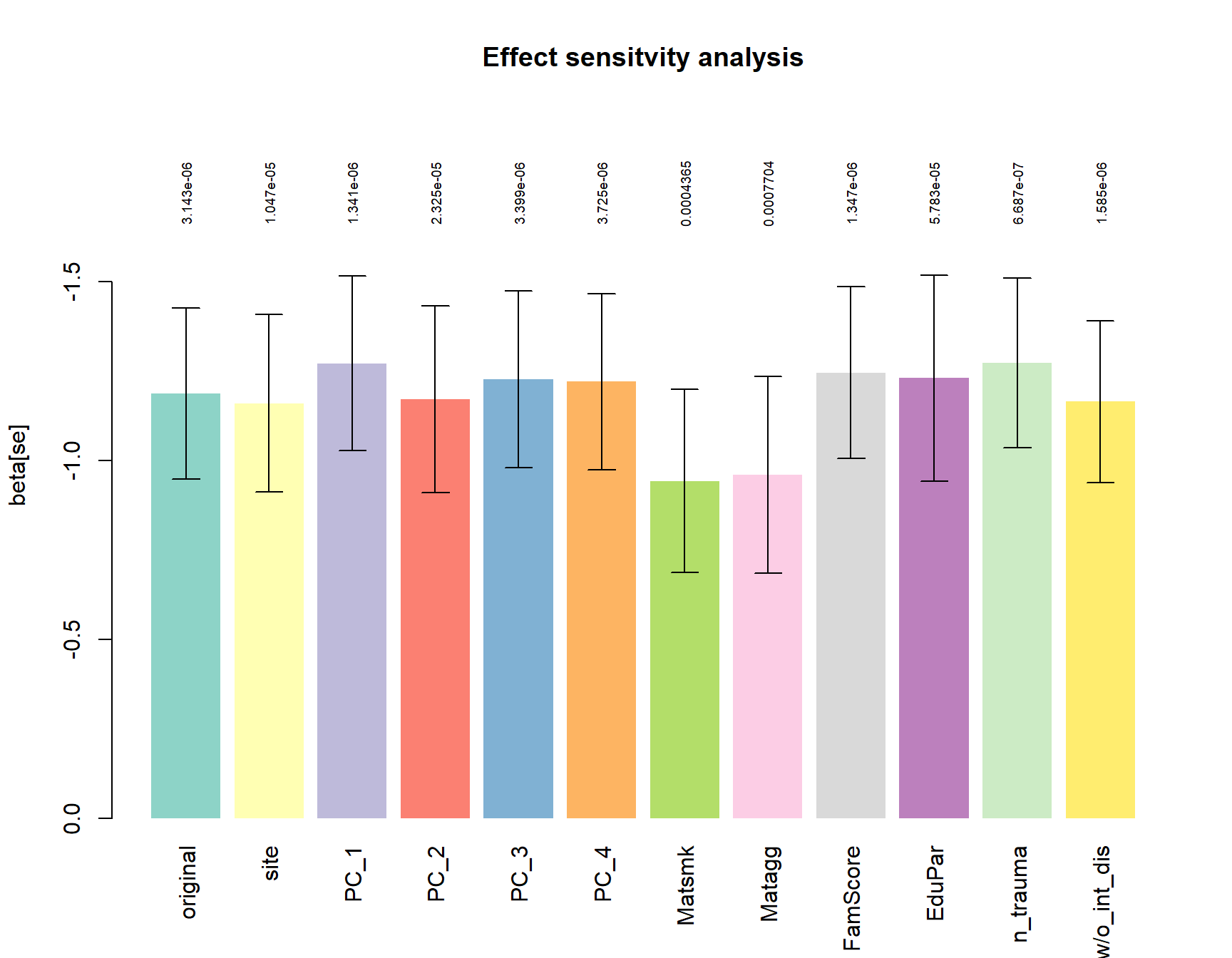
All models are corrected for:
site, Age, Pubstat, int_dis, medication, contraceptives, cigday_1,
site is included as random effect.
original: model defined as 0 + +Age + int_dis + medication + contraceptives + cigday_1 + V8 + group
all other models represent the original model + the variable of interest
Real-time PCR validation
Data loading and parsing
RefGenes = c("GUSB")
Targets_of_Int = c("SLITRK5", "MIR4500HG")
nreplicates = 3
flagscore=Inf #replication quality error
SamplesMeta=read_xlsx(paste0(Home,"/data/RTrawdata/ZelllinienRNA_femNAT.xlsx"))
as.data.frame(SamplesMeta) -> SamplesMeta
SamplesMeta$Pou=paste("POU", SamplesMeta$Pou)
rownames(SamplesMeta)=SamplesMeta$Pou
SamplesMeta$Group = dds_filt$group[match(SamplesMeta$femNATID, dds_filt$ID_femNAT)]
Files=list.files(paste0(Home,"/data/RTrawdata/"), full.names = T)
Files=Files[grepl("_data",Files)]
Sets=unique(substr(basename(Files), 1,8))
Targets_all=vector()
Samples_all=vector()
geoMean=function(x){
x=x[!is.na(x)]
if(length(x)==0)
return(NA)
else
return((prod(x))^(1/length(x)))}
for (Set in Sets){
Setfiles=Files[grep(Set, Files)]
for( i in 1:length(Setfiles)){
tmp=read.table(Setfiles[i], skip=8, header=T, sep="\t", comment.char = "", fill=T)[1:96,]
tmp=tmp[,c("Sample.Name", "Target.Name","CÑ.")]
colnames(tmp)=c("Sample.Name", "Target.Name", "CT")
tmp$Target.Name=gsub("SLITRK5_L", "SLITRK5_", tmp$Target.Name)
tmp$Target.Name=gsub("VD_", "", tmp$Target.Name)
tmp$Target.Name=gsub("_", "", tmp$Target.Name)
tmp$Target.Name=substr(tmp$Target.Name,1, regexpr("#", tmp$Target.Name)-1)
tmp$CT=as.numeric(tmp$CT)
# set bad replicates to NA
tmpmu = tapply(tmp$CT, paste0(tmp$Sample.Name,"_",tmp$Target.Name), mean, na.rm=T)
tmpsd = tapply(tmp$CT, paste0(tmp$Sample.Name,"_",tmp$Target.Name), sd, na.rm=T)
for (corr in which(tmpsd>flagscore)){
index=unlist(strsplit(names(tmpmu)[corr], "_"))
tmp[which(tmp$Sample.Name==index[1] & tmp$Target.Name==index[2]),"CT"] = NA
}
assign(paste0("tmp_",Set,"_",i),tmp)
}
tmp=do.call("rbind", mget(apropos(paste0("tmp_",Set))))
tmp=tmp[which(!(tmp$Sample.Name==""|is.na(tmp$Sample.Name))), ]
tmp=tmp[!tmp$Sample.Name=="NTC",]
Samples=unique(tmp$Sample.Name)
Targets=unique(tmp$Target.Name)
Samples_all=unique(c(Samples_all, Samples))
Targets_all=unique(c(Targets_all, Targets))
Reform=data.frame(matrix(NA, nrow=length(Samples), ncol=length(Targets)*nreplicates))
colnames(Reform)=paste0(rep(Targets, each=3), letters[1:nreplicates])
rownames(Reform)=Samples
for (i in Samples) {
#print(i)
for (j in Targets){
Reform[i,grep(j, colnames(Reform))]=tmp[tmp$Sample.Name==i & tmp$Target.Name==j,"CT"]
}
}
HK=colnames(Reform)[grep(paste0(RefGenes, collapse="|"),colnames(Reform))]
GMHK=apply(Reform[,HK], 1, geoMean)
tmp2=Reform-GMHK
assign(paste0(Set,"_dCT"), tmp2)
rm(list=c(apropos("tmp"), "Reform", "GMHK"))
}Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenWarning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenWarning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenWarning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenWarning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenWarning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenWarning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenWarning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugt
Warning: NAs durch Umwandlung erzeugtWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'get_java_tmp_dir' nicht gefundenWarning in rm(list = c(apropos("tmp"), "Reform", "GMHK")): Objekt
'set_java_tmp_dir' nicht gefundenSamples_all=unique(Samples_all)
Targets_all = unique(Targets_all)
mergedCTtable=data.frame(matrix(NA,ncol=length(Targets_all)*nreplicates, nrow=length(Samples_all)))
colnames(mergedCTtable)=paste0(rep(unique(Targets_all), each=nreplicates), letters[1:nreplicates])
rownames(mergedCTtable)=Samples_all
CTobj=apropos("_dCT")
for( obj in CTobj){
DF=get(obj)
for(k in colnames(DF)){
for(l in rownames(DF)){
mergedCTtable[l,k]=DF[l,k]
}
}
}
CTmeans=colMeans(mergedCTtable, na.rm = T)
meanvec=tapply(CTmeans,gsub(paste0(letters[1:nreplicates],collapse="|"),"",names(CTmeans)), mean, na.rm=T)
meanvec = rep(meanvec, each=nreplicates)
names(meanvec) = paste0(names(meanvec), letters[1:nreplicates])
meanvec=meanvec[colnames(mergedCTtable)]
ddCT=apply(mergedCTtable,1, function(x){x-meanvec})
FC=2^-ddCT
SamplesMeta$inset=F
SamplesMeta$inset[SamplesMeta$Pou %in% colnames(FC)]=T
SamplesMeta=SamplesMeta[SamplesMeta$inset,]
CTRLCASEsorter=c(which(SamplesMeta$Group=="CTRL"),which(SamplesMeta$Group=="CD"))
SamplesMeta = SamplesMeta[CTRLCASEsorter, ]
searcher=paste0(Targets_of_Int, collapse = "|")
FC = FC[grepl(searcher, rownames(FC)),SamplesMeta$Pou]
MuFC=apply(FC, 2, function(x){tapply(log2(x), gsub("a|b|c","",rownames(FC)), mean, na.rm=T)})
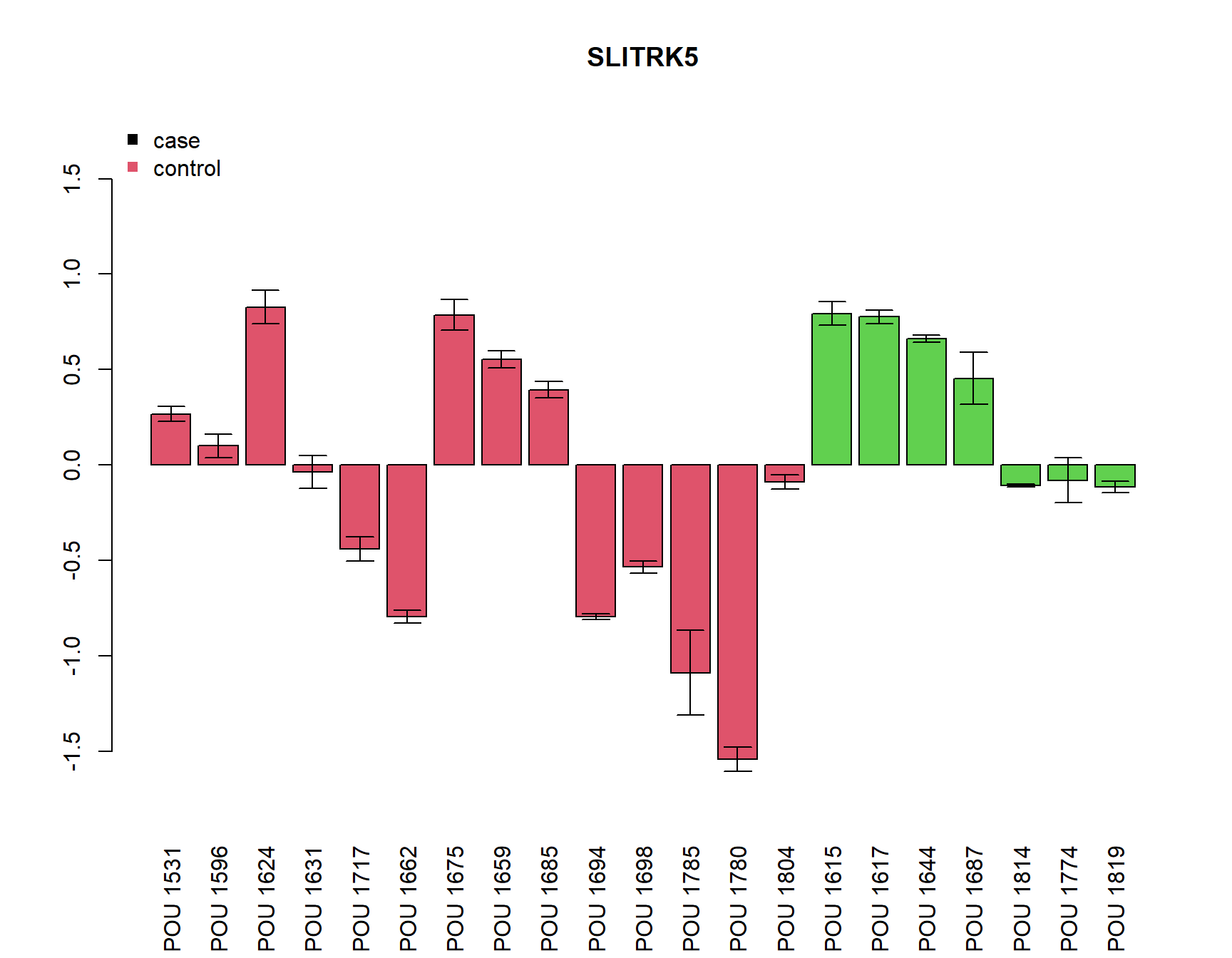
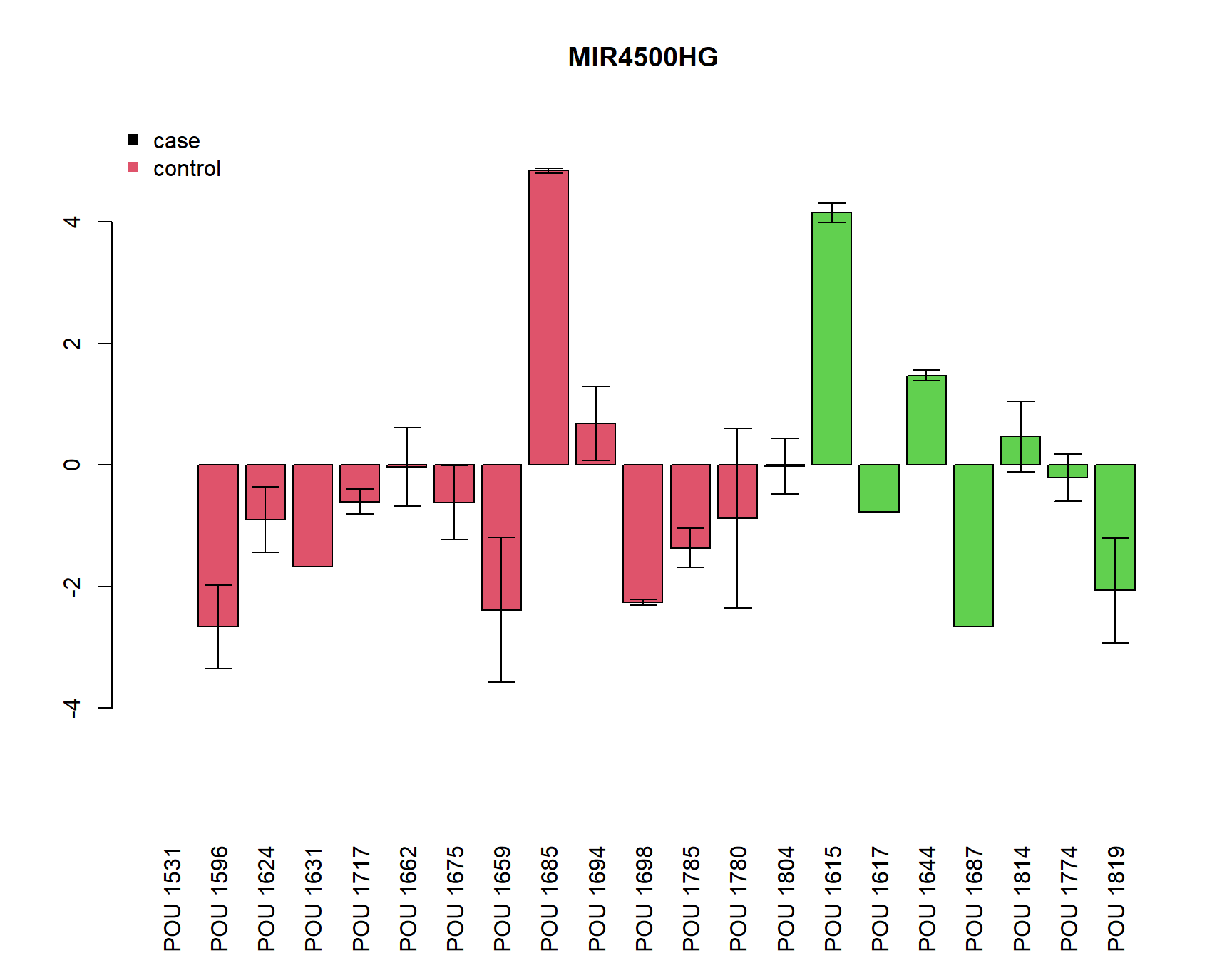
SDFC=apply(FC, 2, function(x){tapply(log2(x), gsub("a|b|c","",rownames(FC)), sd, na.rm=T)})plot relative expression by samples
pdf(paste0(Home, "/output/barplots.pdf"))
for(i in Targets_of_Int){
if(any(!is.na(MuFC[i,]))){
a=barplot(unlist(MuFC[i,]), col=as.numeric(SamplesMeta[colnames(MuFC),"Group"])+1, main=i, las=3,
ylim=c(-max(abs(MuFC[i,])*1.2, na.rm=T), (max(abs(MuFC[i,])*1.2, na.rm=T))))
arrows(a, MuFC[i,], a, MuFC[i,]+SDFC[i,], angle = 90, length = 0.1)
arrows(a, MuFC[i,], a, MuFC[i,]-SDFC[i,], angle = 90, length = 0.1)
legend("topleft", c("case", "control"), col=c(1,2), pch=15, bty="n")
} else {
plot(0,0, type="n", main=paste(i, "not detected"))
}
}
dev.off()png
2 for(i in Targets_of_Int){
if(any(!is.na(MuFC[i,]))){
a=barplot(unlist(MuFC[i,]), col=as.numeric(SamplesMeta[colnames(MuFC),"Group"])+1, main=i, las=3,
ylim=c(-max(abs(MuFC[i,])*1.2, na.rm=T), (max(abs(MuFC[i,])*1.2, na.rm=T))))
arrows(a, MuFC[i,], a, MuFC[i,]+SDFC[i,], angle = 90, length = 0.1)
arrows(a, MuFC[i,], a, MuFC[i,]-SDFC[i,], angle = 90, length = 0.1)
legend("topleft", c("case", "control"), col=c(1,2), pch=15, bty="n")
} else {
plot(0,0, type="n", main=paste(i, "not detected"))
}
}
compare across groups
sink(paste0(Home, "/output/ResultsgroupComp.txt"))
Group=SamplesMeta$Group
for(i in Targets_of_Int){
print(i)
print(summary(try(lm(unlist(MuFC[i,])~Group))))
print(t.test(unlist(MuFC[i,])~Group))
}[1] "SLITRK5"
Call:
lm(formula = unlist(MuFC[i, ]) ~ Group)
Residuals:
Min 1Q Median 3Q Max
-1.3710 -0.4489 0.1142 0.4382 0.9983
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) -0.1703 0.1718 -0.991 0.334
GroupCD 0.5109 0.2975 1.717 0.102
Residual standard error: 0.6427 on 19 degrees of freedom
Multiple R-squared: 0.1343, Adjusted R-squared: 0.08878
F-statistic: 2.949 on 1 and 19 DF, p-value: 0.1022
Welch Two Sample t-test
data: unlist(MuFC[i, ]) by Group
t = -2.0316, df = 18.197, p-value = 0.05706
alternative hypothesis: true difference in means is not equal to 0
95 percent confidence interval:
-1.03871726 0.01701686
sample estimates:
mean in group CTRL mean in group CD
-0.1702834 0.3405668
[1] "MIR4500HG"
Call:
lm(formula = unlist(MuFC[i, ]) ~ Group)
Residuals:
Min 1Q Median 3Q Max
-2.7172 -1.2116 -0.2660 0.5749 5.4474
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) -0.6053 0.5683 -1.065 0.301
GroupCD 0.6614 0.9607 0.688 0.500
Residual standard error: 2.049 on 18 degrees of freedom
(1 observation deleted due to missingness)
Multiple R-squared: 0.02566, Adjusted R-squared: -0.02847
F-statistic: 0.474 on 1 and 18 DF, p-value: 0.4999
Welch Two Sample t-test
data: unlist(MuFC[i, ]) by Group
t = -0.65047, df = 10.602, p-value = 0.5292
alternative hypothesis: true difference in means is not equal to 0
95 percent confidence interval:
-2.909646 1.586853
sample estimates:
mean in group CTRL mean in group CD
-0.60525003 0.05614653 sink()
SamplesMeta$femNATID2=paste0("ID_",gsub("-","_",SamplesMeta$femNATID))
SamplesMeta=SamplesMeta[SamplesMeta$Pou %in% colnames(MuFC),]
MuFC=MuFC[,SamplesMeta$Pou]
TPM4RNA=selEpitpm[,SamplesMeta$femNATID2]
colnames(TPM4RNA)=SamplesMeta$Pou
tags=list()
Targets=Targets_of_Int
sigtags=which(restab$padj<=0.05)
tagsOI=grep(paste0(Targets, collapse = "|"),selEpiMeta$gene)
sigtagsOI = tagsOI[tagsOI %in% sigtags]
fintagsOI=data.frame(tags=sigtagsOI, gene=selEpiMeta[sigtagsOI,"gene"])
#Targ=Targets[1]
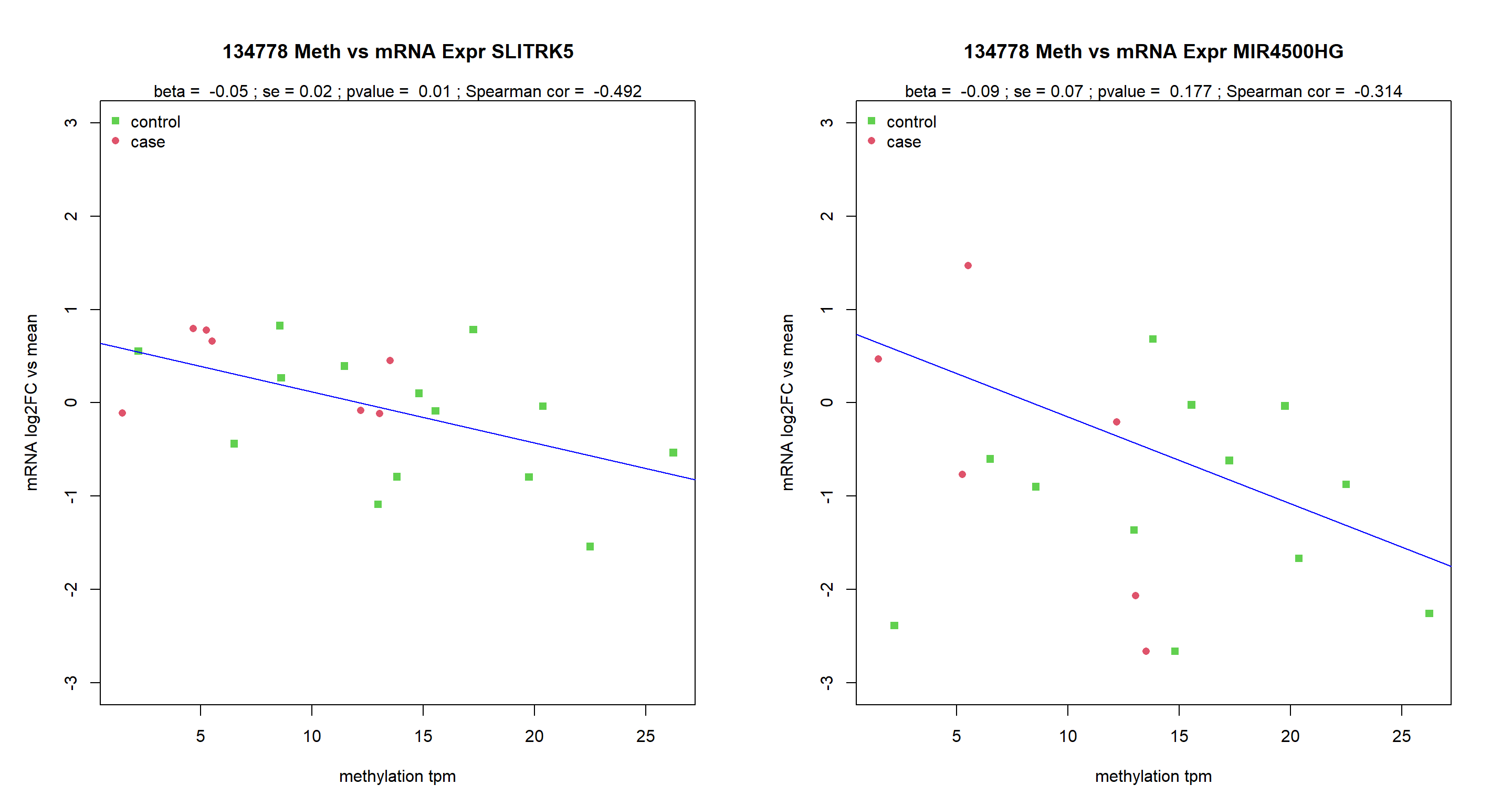
#tag=tags[1]compare against methylation level
pdf(paste0(Home,"/output/RNAvsMETplots.pdf"), width = 15, height = 8)
MuFC=as.data.frame(MuFC)
MuFCsel=MuFC[Targets,]
par(mar=c(5,5,5,3), mfrow=c(1,2))
for (Targ in Targets){
tags=fintagsOI$tags[grep(Targ, fintagsOI$gene)]
for (tag in tags){
data=data.frame(tpm=unlist(TPM4RNA[tag,SamplesMeta$Pou]) ,
RT=unlist(MuFCsel[grep(Targ, rownames(MuFCsel)),SamplesMeta$Pou]))
plot(data$tpm,data$RT,
xlab="methylation tpm",
ylab = "mRNA log2FC vs mean",
ylim=c(-3,3),
col=4-as.numeric(SamplesMeta$Group),
pch=as.numeric(SamplesMeta$Group)+14,
main=paste(tag, "Meth vs mRNA Expr", Targ))
legend("topleft", c("control", "case"), pch=c(15,16), col=c(3,2), bty="n")
a=lm(RT~tpm, data)
b=summary(a)
abline(a, col="blue")
SperCor=cor(data$RT,data$tpm,use = "c", method = "spearman")
mtext(3, text = paste("beta = ", round(coefficients(a)[2],2),
"; se =", round(b$coefficients[2,2],2),
"; pvalue = ", round(b$coefficients[2,4],3),
"; sperman cor = ", round(SperCor,3)))
}
}
dev.off()png
2 MuFC=as.data.frame(MuFC)
MuFCsel=MuFC[Targets,]
par(mar=c(5,5,5,3), mfrow=c(1,2))
for (Targ in Targets){
tags=fintagsOI$tags[grep(Targ, fintagsOI$gene)]
for (tag in tags){
data=data.frame(tpm=unlist(TPM4RNA[tag,SamplesMeta$Pou]) ,
RT=unlist(MuFCsel[grep(Targ, rownames(MuFCsel)),SamplesMeta$Pou]))
plot(data$tpm,data$RT,
xlab="methylation tpm",
ylab = "mRNA log2FC vs mean",
ylim=c(-3,3),
col=4-as.numeric(SamplesMeta$Group),
pch=as.numeric(SamplesMeta$Group)+14,
main=paste(tag, "Meth vs mRNA Expr", Targ))
legend("topleft", c("control", "case"), pch=c(15,16), col=c(3,2), bty="n")
a=lm(RT~tpm, data)
b=summary(a)
abline(a, col="blue")
SperCor=cor(data$RT,data$tpm,use = "c", method = "spearman")
mtext(3, text = paste("beta = ", round(coefficients(a)[2],2),
"; se =", round(b$coefficients[2,2],2),
"; pvalue = ", round(b$coefficients[2,4],3),
"; Spearman cor = ", round(SperCor,3)))
}
}
Systems Analysis
Genomic feature enrichment
Significant loci with a p-value <= 0.01 and a absolute log2 fold-change lager 0.5 were tested for enrichment in annotated genomic feature using fisher exact test.
Ranges=rowData(dds_filt)
TotTagsofInterest=sum(Ranges$WaldPvalue_groupCD<=thresholdp & abs(Ranges$groupCD)>thresholdLFC)
Resall=data.frame()
index = Ranges$WaldPvalue_groupCD<=thresholdp& abs(Ranges$groupCD)>thresholdLFC
for (feat in unique(Ranges$feature)){
tmp=table(Ranges$feature == feat, signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resall = rbind(Resall, res)
}
tmp=table(Ranges$tf_binding!="", signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resall = rbind(Resall, res)
tmp=table(Ranges$cpg=="cpg", signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resall = rbind(Resall, res)
colnames(Resall)=c("OR", "CI95L", "CI95U", "P")
rownames(Resall)=c(unique(Ranges$feature), "TF-binding", "CpG-island")
Resall$Beta = log(Resall$OR)
Resall$SE = (log(Resall$OR)-log(Resall$CI95L))/1.96
Resall$Padj=p.adjust(Resall$P, method = "bonferroni")
Resdown=data.frame()
index = Ranges$WaldPvalue_groupCD<=thresholdp & Ranges$groupCD<thresholdLFC
for (feat in unique(Ranges$feature)){
tmp=table(Ranges$feature == feat, signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resdown = rbind(Resdown, res)
}
tmp=table(Ranges$tf_binding!="", signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resdown = rbind(Resdown, res)
tmp=table(Ranges$cpg=="cpg", signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resdown = rbind(Resdown, res)
colnames(Resdown)=c("OR", "CI95L", "CI95U", "P")
rownames(Resdown)=c(unique(Ranges$feature), "TF-binding", "CpG-island")
Resdown$Beta = log(Resdown$OR)
Resdown$SE = (log(Resdown$OR)-log(Resdown$CI95L))/1.96
Resdown$Padj=p.adjust(Resdown$P, method = "bonferroni")
Resup=data.frame()
index = Ranges$WaldPvalue_groupCD<=thresholdp & Ranges$groupCD>thresholdLFC
for (feat in unique(Ranges$feature)){
tmp=table(Ranges$feature == feat, signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resup = rbind(Resup, res)
}
tmp=table(Ranges$tf_binding!="", signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resup = rbind(Resup, res)
tmp=table(Ranges$cpg=="cpg", signif=index)
resfish=fisher.test(tmp)
res = c(resfish$estimate, unlist(resfish$conf.int), resfish$p.value)
Resup = rbind(Resup, res)
colnames(Resup)=c("OR", "CI95L", "CI95U", "P")
rownames(Resup)=c(unique(Ranges$feature), "TF-binding", "CpG-island")
Resup$Beta = log(Resup$OR)
Resup$SE = (log(Resup$OR)-log(Resup$CI95L))/1.96
Resup$Padj=p.adjust(Resup$P, method = "bonferroni")
multiORplot(Resall, Pval = "P", Padj = "Padj", beta="Beta",SE = "SE", pheno="All diff. methylated loci")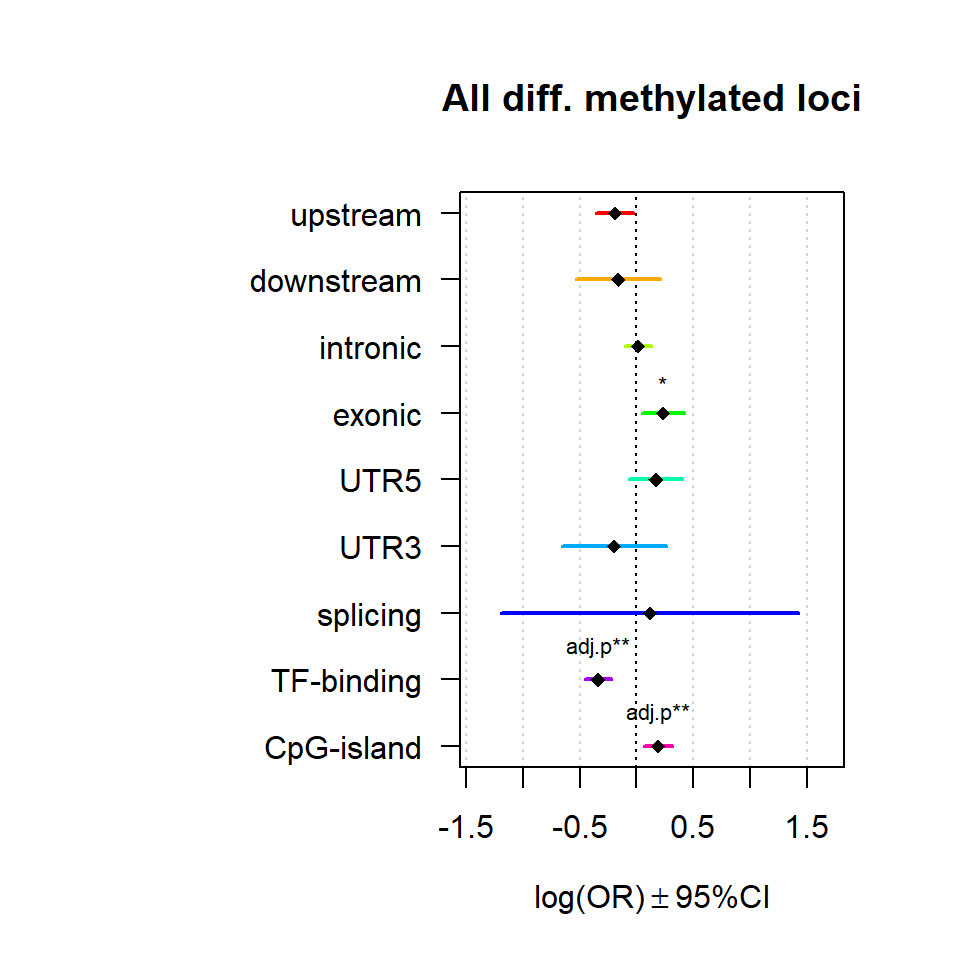
multiORplot(Resup, Pval = "P", Padj = "Padj", beta="Beta",SE = "SE", pheno="hypomethylated loci")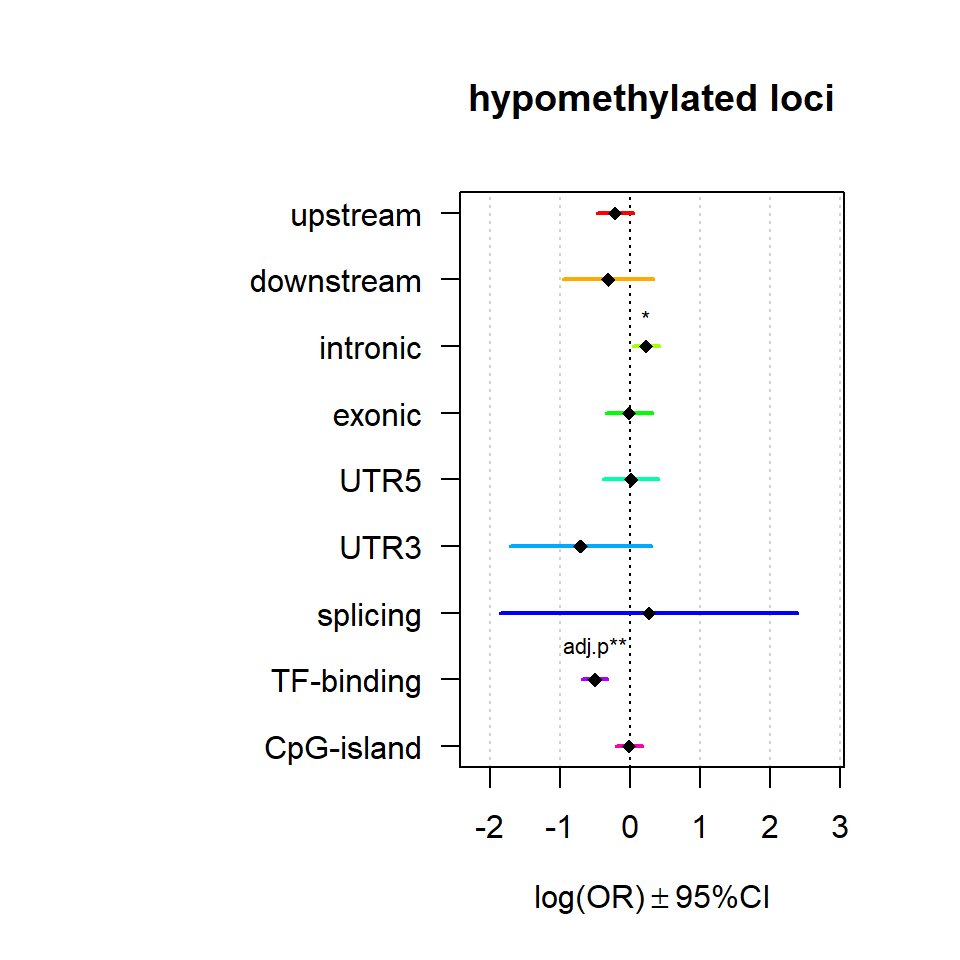
multiORplot(Resdown, Pval = "P", Padj = "Padj", beta="Beta",SE = "SE", pheno="Hypermethylated loci")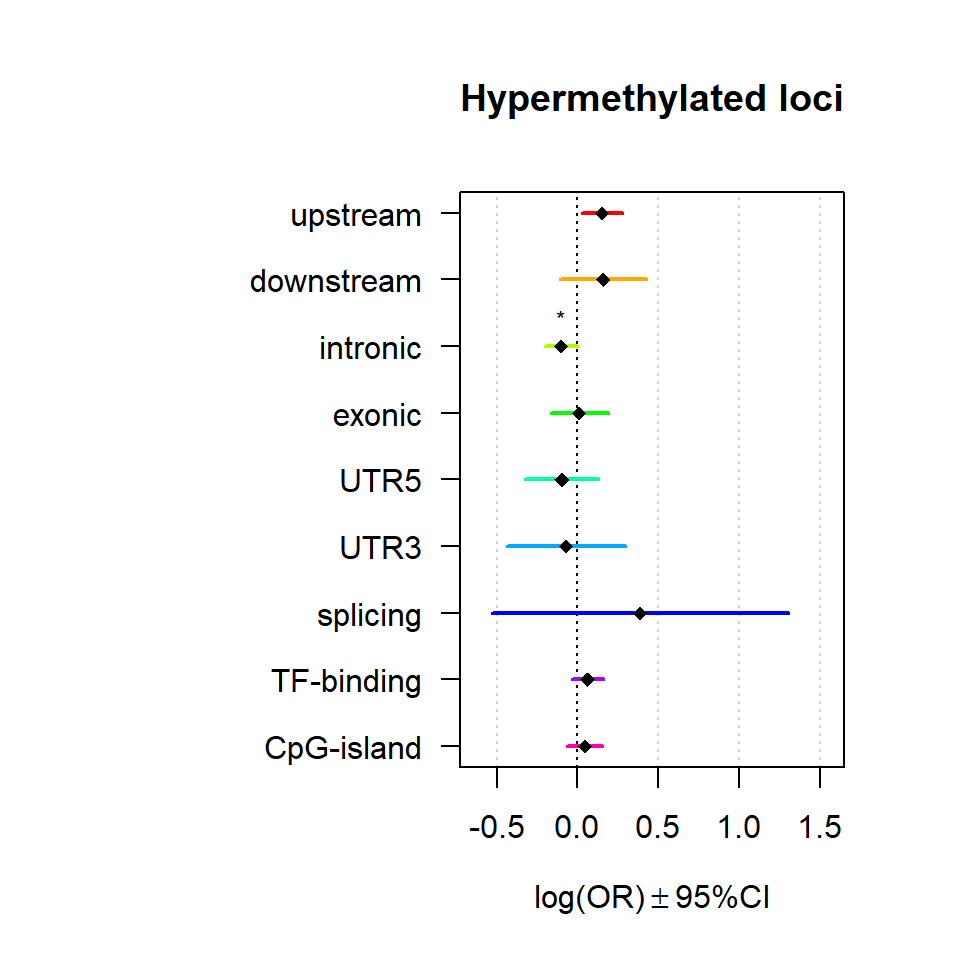
pdf(paste0(Home, "/output/functional_Enrichemnt.pdf"))
multiORplot(Resall, Pval = "P", Padj = "Padj", beta="Beta",SE = "SE", pheno="All diff. methylated loci")
multiORplot(Resup, Pval = "P", Padj = "Padj", beta="Beta",SE = "SE", pheno="hypomethylated loci")
multiORplot(Resdown, Pval = "P", Padj = "Padj", beta="Beta",SE = "SE", pheno="Hypermethylated loci")
dev.off()png
2 GO-term Enrichment
Significant loci and differentially methylated regions with a p-value <= 0.01 and an absolute log2 fold-change lager 0.5 were tested for enrichment among GO-terms Molecular Function, Cellular Compartment and Biological Processes, KEGG pathways, Transcription factor Binding sites, Human Protein Atlas Tissue Expression, Human Phenotypes.
getGOresults = function(geneset, genereference){
resgo = gost(geneset, organism = "hsapiens",
correction_method = "g_SCS",
domain_scope = "custom",
sources = c("GO:BP", "GO:MF", "GO:CC"),
custom_bg = genereference)
if(length(resgo) != 0){
return(resgo)
} else {
print("no significant results")
return(NULL)
}
}
gene_univers = getuniquegenes(as.data.frame(rowRanges(dds_filt))$gene)
idx = (results_Deseq$pvalue <= thresholdp &
(abs(results_Deseq$log2FoldChange) > thresholdLFC))
genes_reg = getuniquegenes(as.data.frame(rowRanges(dds_filt))$gene[idx])
dmr_genes = unique(resultsdmr_table$name[resultsdmr_table$p.value<=thresholdp &
abs(resultsdmr_table$value)>=thresholdLFC])
Genes_of_interset = list("01_dmregions" = dmr_genes,
"02_dmtag" = genes_reg
)
gostres = getGOresults(Genes_of_interset, gene_univers)
gostplot(gostres, capped = TRUE, interactive = T)p = gostplot(gostres, capped = TRUE, interactive = F)
toptab = gostres$result
pp = publish_gostplot(p, filename = paste0(Home,"/output/gostres.pdf"))The image is saved to C:/Users/chiocchetti/Projects/femNATCD_MethSeq/output/gostres.pdfwrite.xlsx2(toptab, file = paste0(Home,"/output/GOres.xlsx"), sheetName = "GO_enrichment")Brain Developmental Processes Enrichment tests
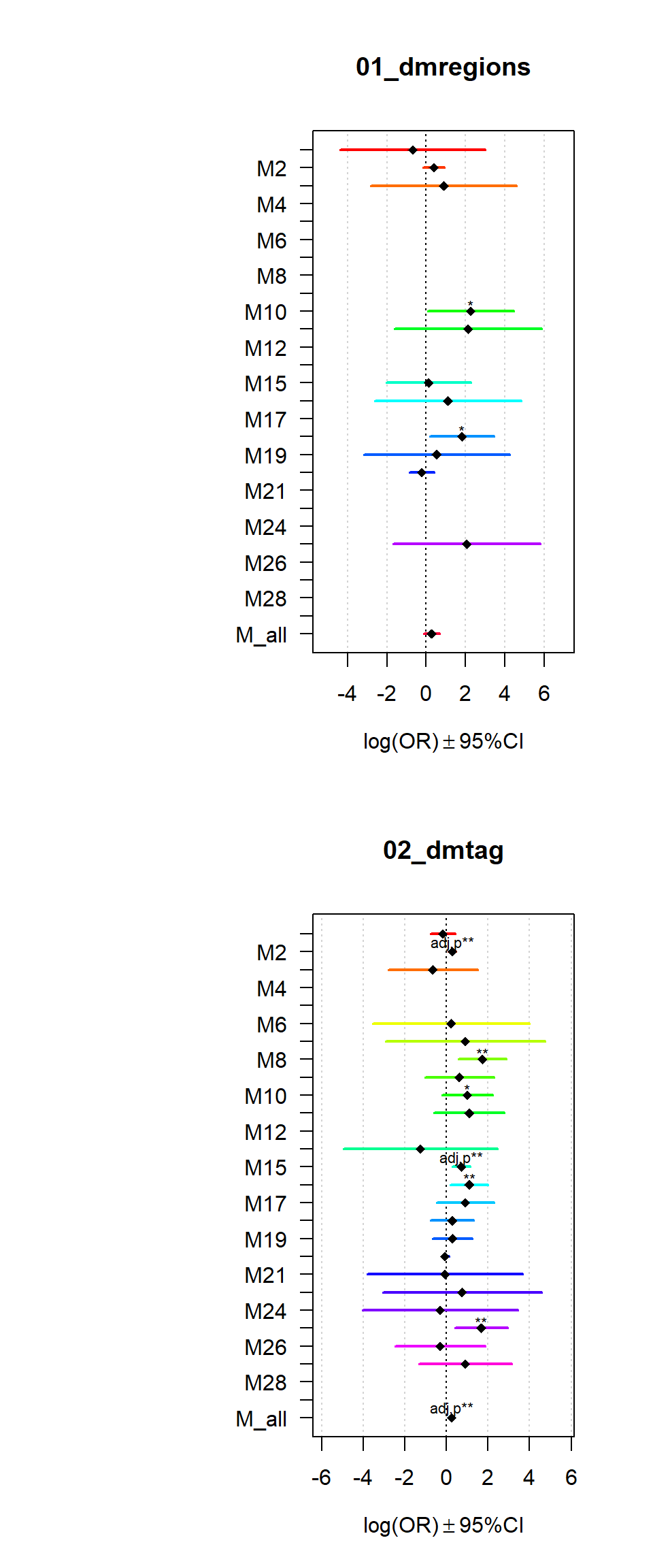
Gene sets identified to be deferentially methylated with a p-value <= 0.01 and an absolute log2 fold-change larger 0.5 were tested for enrichment among gene-modules coregulated during Brain expression.
Kang Modules
# define Reference Universe
KangUnivers<- read.table(paste0(Home,"/data/KangUnivers.txt"), sep="\t", header=T)
colnames(KangUnivers)<-c("EntrezId","Symbol")
Kang_genes<-read.table(paste0(Home,"/data/Kang_dataset_genesMod_version2.txt"),sep="\t",header=TRUE)
#3)Generate Gene universe to be used for single gene lists
tmp=merge(KangUnivers,Kang_genes,by.y="EntrezGene",by.x="EntrezId",all=TRUE) #18826
KangUni_Final<-tmp[duplicated(tmp$EntrezId)==FALSE,] #18675
# Local analysis gene universe
Annotation_list<-data.frame(Symbol = gene_univers)
# match modules
Annotation_list$Module = Kang_genes$Module[match(Annotation_list$Symbol,Kang_genes$symbol)]
# check if overlapping in gene universes
Annotation_list$univers = Annotation_list$Symbol %in% KangUni_Final$Symbol
# drop duplicates
Annotation_list = Annotation_list[duplicated(Annotation_list$Symbol)==FALSE,]
# selct only genes that have been detected on both datasets
Annotation_list = Annotation_list[Annotation_list$univers==T,]
# final reference
UniversalGeneset=Annotation_list$Symbol
# define Gene lists to test
# sort and order Modules to be tested
Modules=unique(Annotation_list$Module)
Modules = Modules[! Modules %in% c(NA, "")]
Modules = Modules[order(as.numeric(gsub("M","",Modules)))]
GL_all=list()
for(i in Modules){
GL_all[[i]]=Annotation_list$Symbol[Annotation_list$Module%in%i]
}
GL_all[["M_all"]]=Kang_genes$symbol[Kang_genes$Module %in% Modules]
GOI1 = Genes_of_interset
Resultsall=list()
for(j in names(GOI1)){
Res = data.frame()
for(i in names(GL_all)){
Modulegene=GL_all[[i]]
Factorgene=GOI1[[j]]
Testframe<-fisher.test(table(factor(UniversalGeneset %in% Factorgene,levels=c("TRUE","FALSE")),
factor(UniversalGeneset %in% Modulegene,levels=c("TRUE","FALSE"))))
beta=log(Testframe$estimate)
Res[i, "beta"] =beta
Res[i, "SE"]=abs(beta-log(Testframe$conf.int[1]))/1.96
Res[i, "Pval"]=Testframe$p.value
Res[i, "OR"]=(Testframe$estimate)
Res[i, "ORL"]=(Testframe$conf.int[1])
Res[i, "ORU"]=(Testframe$conf.int[2])
}
Res$Padj = p.adjust(Res$Pval, method = "bonferroni")
Resultsall[[j]] = Res
}
par(mfrow = c(2,1))
for (i in names(Resultsall)){
multiORplot(datatoplot = Resultsall[[i]], pheno=i)
}
par(mfrow = c(1,1))
pdf(paste0(Home, "/output/BrainMod_Enrichemnt.pdf"))
for (i in names(Resultsall)){
multiORplot(datatoplot = Resultsall[[i]], pheno=i)
}
dev.off()png
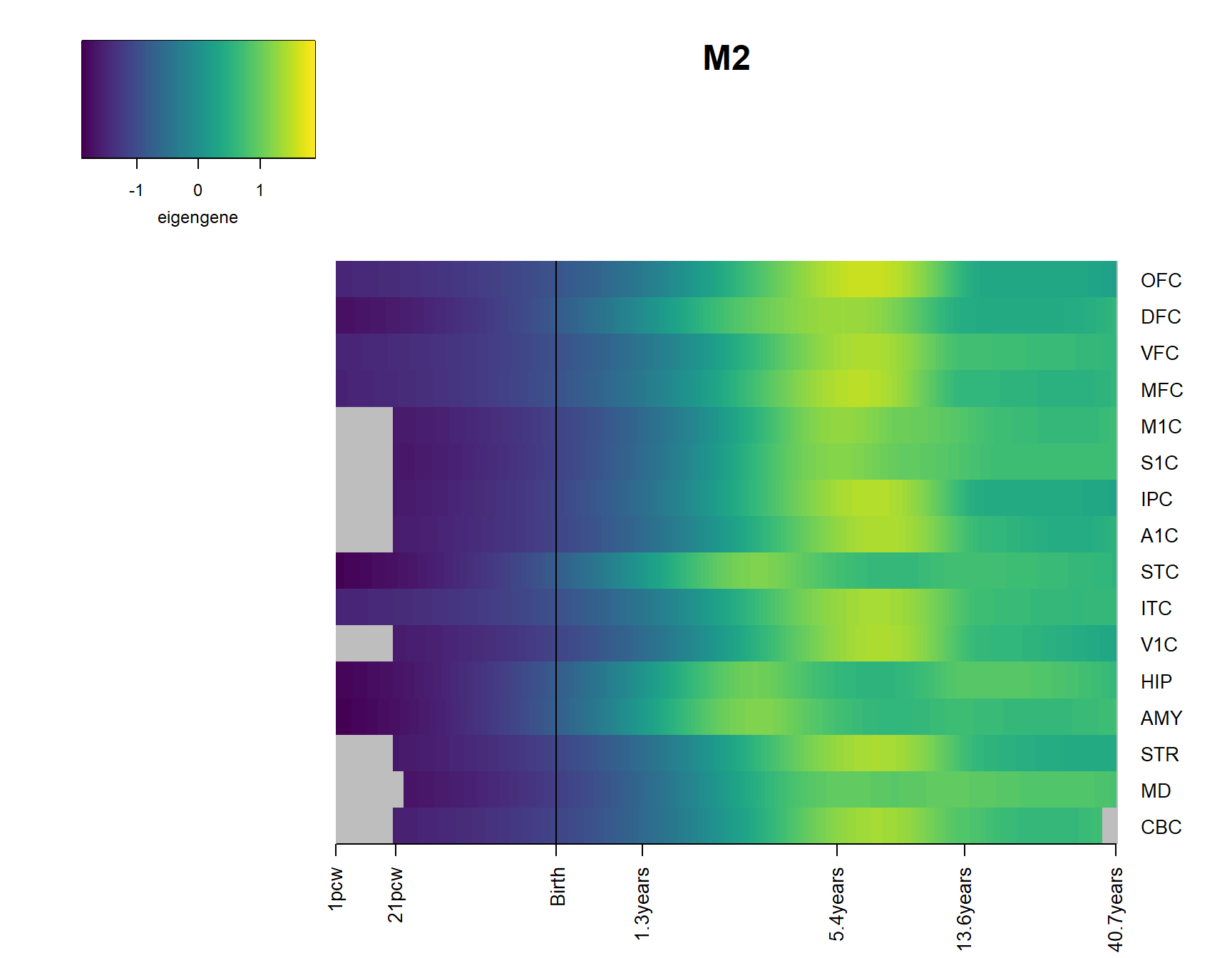
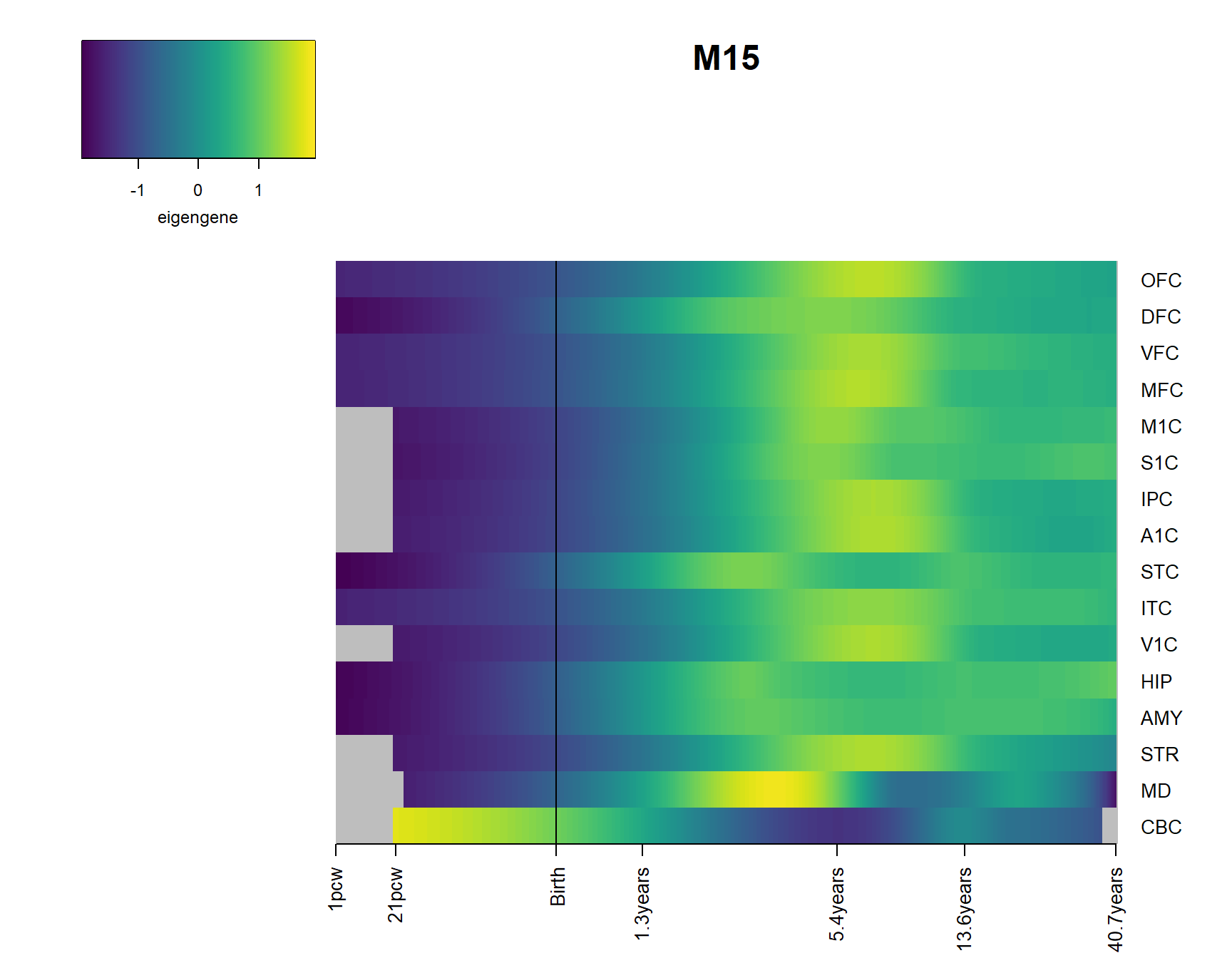
2 Modsig = c()
for(r in names(Resultsall)){
a=rownames(Resultsall[[r]])[Resultsall[[r]]$Padj<=0.05]
Modsig = c(Modsig,a)
}Brain espresseion heatmaps
# show brains and expression
Modsig2=unique(Modsig[Modsig!="M_all"])
load(paste0(Home,"/data/Kang_DataPreprocessing.RData")) #Load the Kang expression data of all genes
datExprPlot=matriz #Expression data of Kang loaded as Rdata object DataPreprocessing.RData
Genes = GL_all[names(GL_all)!="M_all"]
Genes_expression<-list()
pcatest<-list()
for (i in names(Genes)){
Genes_expression[[i]]<-matriz[,which(colnames(matriz) %in% Genes[[i]])]
pcatest[[i]]=prcomp(t(as.matrix(Genes_expression[[i]])),retx=TRUE)
}
# PCA test
PCA<-data.frame(pcatest[[1]]$rotation)
PCA$donor_name<-rownames(PCA)
PC1<-data.frame(PCA[,c(1,ncol(PCA))])
#Combining the age with expression data
list <- strsplit(sampleInfo$age, " ")
library("plyr")------------------------------------------------------------------------------You have loaded plyr after dplyr - this is likely to cause problems.
If you need functions from both plyr and dplyr, please load plyr first, then dplyr:
library(plyr); library(dplyr)------------------------------------------------------------------------------
Attache Paket: 'plyr'The following object is masked from 'package:matrixStats':
countThe following object is masked from 'package:IRanges':
descThe following object is masked from 'package:S4Vectors':
renameThe following objects are masked from 'package:dplyr':
arrange, count, desc, failwith, id, mutate, rename, summarise,
summarizeThe following object is masked from 'package:purrr':
compactdf <- ldply(list)
colnames(df) <- c("Age", "time")
sampleInfo<-cbind(sampleInfo[,1:9],df)
sampleInfo$Age<-as.numeric(sampleInfo$Age)
sampleInfo$period<-ifelse(sampleInfo$time=="pcw",sampleInfo$Age*7,ifelse(sampleInfo$time=="yrs",sampleInfo$Age*365+270,ifelse(sampleInfo$time=="mos",sampleInfo$Age*30+270,NA)))
#We need it just for the donor names
PCA_matrix<-merge.with.order(PC1,sampleInfo,by.y="SampleID",by.x="donor_name",keep_order=1)
#Select which have phenotype info present
matriz2<-matriz[which(rownames(matriz) %in% PCA_matrix$donor_name),]
FactorGenes_expression<-list()
#Factors here mean modules
for (i in names(Genes)){
FactorGenes_expression[[i]]<-matriz2[,which(colnames(matriz2) %in% Genes[[i]])]
}
FactorseGE<-list()
for (i in names(Genes)){
FactorseGE[[i]]<-FactorGenes_expression[[i]]
}
allModgenes=NULL
colors=vector()
for ( i in names(Genes)){
allModgenes=cbind(allModgenes,FactorseGE[[i]])
colors=c(colors, rep(i, ncol(FactorseGE[[i]])))
}
lengths=unlist(lapply(FactorGenes_expression, ncol), use.names = F)
MEorig=moduleEigengenes(allModgenes, colors)
PCA_matrixfreeze=PCA_matrix
index=!PCA_matrix$structure_acronym %in% c("URL", "DTH", "CGE","LGE", "MGE", "Ocx", "PCx", "M1C-S1C","DIE", "TCx", "CB")
PCA_matrix=PCA_matrix[index,]
ME = MEorig$eigengenes[index,]
matsel = matriz2[index,]
colnames(ME) = gsub("ME", "", colnames(ME))
timepoints=seq(56,15000, length.out=1000)
matrix(c("CB", "THA", "CBC", "MD"), ncol=2 ) -> cnm
brainheatmap=function(Module){
MEmod=ME[,Module]
toplot=data.frame(matrix(NA, nrow=length(table(PCA_matrix$structure_acronym)), ncol=998))
rownames(toplot)=unique(PCA_matrix$structure_acronym)
target <- c("OFC", "DFC", "VFC", "MFC","M1C","S1C","IPC","A1C","STC","ITC","V1C","HIP","AMY","STR","MD","CBC")
toplot<-toplot[c(6,2,8,5,11,12,10,9,7,4,14,3,1,13,16,15),]
for ( i in unique(PCA_matrix$structure_acronym)){
index=PCA_matrix$structure_acronym==i
LOESS=loess(MEmod[index]~PCA_matrix$period[index])
toplot[i,]=predict(LOESS,newdata = round(exp(seq(log(56),log(15000), length.out=998)),2))
colnames(toplot)[c(1,77,282,392,640,803,996)]<-
c("1pcw","21pcw","Birth","1.3years","5.4years","13.6years","40.7years")
}
cols=viridis(100)
labvec <- c(rep(NA, 1000))
labvec[c(1,77,282,392,640,803,996)] <- c("1pcw","21pcw","Birth","1.3years","5.4years","13.6years","40.7years")
toplot<-toplot[,1:998]
date<-c(1:998)
dateY<-paste0(round(date/365,2),"_Years")
names(toplot)<-dateY
par(xpd=FALSE)
heatmap.2(as.matrix(toplot), col = cols,
main=Module,
trace = "none",
na.color = "grey",
Colv = F, Rowv = F,
labCol = labvec,
#breaks = seq(-0.1,0.1, length.out=101),
symkey = T,
scale = "row",
key.title = "",
dendrogram = "none",
key.xlab = "eigengene",
density.info = "none",
#main=paste("Module",1),
srtCol=90,
tracecol = "none",
cexRow = 1,
add.expr=eval.parent(abline(v=282),
axis(1,at=c(1,77,282,392,640,803,996),
labels =FALSE)),cexCol = 1)
}
brainheatmap_gene=function(Genename){
MEmod=matsel[,Genename]
toplot=data.frame(matrix(NA, nrow=length(table(PCA_matrix$structure_acronym)), ncol=998))
rownames(toplot)=unique(PCA_matrix$structure_acronym)
target <- c("OFC", "DFC", "VFC", "MFC","M1C","S1C","IPC","A1C","STC","ITC","V1C","HIP","AMY","STR","MD","CBC")
toplot<-toplot[c(6,2,8,5,11,12,10,9,7,4,14,3,1,13,16,15),]
for ( i in unique(PCA_matrix$structure_acronym)){
index=PCA_matrix$structure_acronym==i
LOESS=loess(MEmod[index]~PCA_matrix$period[index])
toplot[i,]=predict(LOESS,newdata = round(exp(seq(log(56),log(15000), length.out=998)),2))
colnames(toplot)[c(1,77,282,392,640,803,996)]<-
c("1pcw","21pcw","Birth","1.3years","5.4years","13.6years","40.7years")
}
cols=viridis(100)
labvec <- c(rep(NA, 1000))
labvec[c(1,77,282,392,640,803,996)] <- c("1pcw","21pcw","Birth","1.3years","5.4years","13.6years","40.7years")
toplot<-toplot[,1:998]
date<-c(1:998)
dateY<-paste0(round(date/365,2),"_Years")
names(toplot)<-dateY
par(xpd=FALSE)
heatmap.2(as.matrix(toplot), col = cols,
main=Genename,
trace = "none",
na.color = "grey",
Colv = F, Rowv = F,
labCol = labvec,
#breaks = seq(-0.1,0.1, length.out=101),
symkey = F,
scale = "none",
key.title = "",
dendrogram = "none",
key.xlab = "eigengene",
density.info = "none",
#main=paste("Module",1),
#srtCol=90,
tracecol = "none",
cexRow = 1,
add.expr=eval.parent(abline(v=282),
axis(1,at=c(1,77,282,392,640,803,996),
labels =FALSE))
,cexCol = 1)
}
brainheatmap_gene("SLITRK5")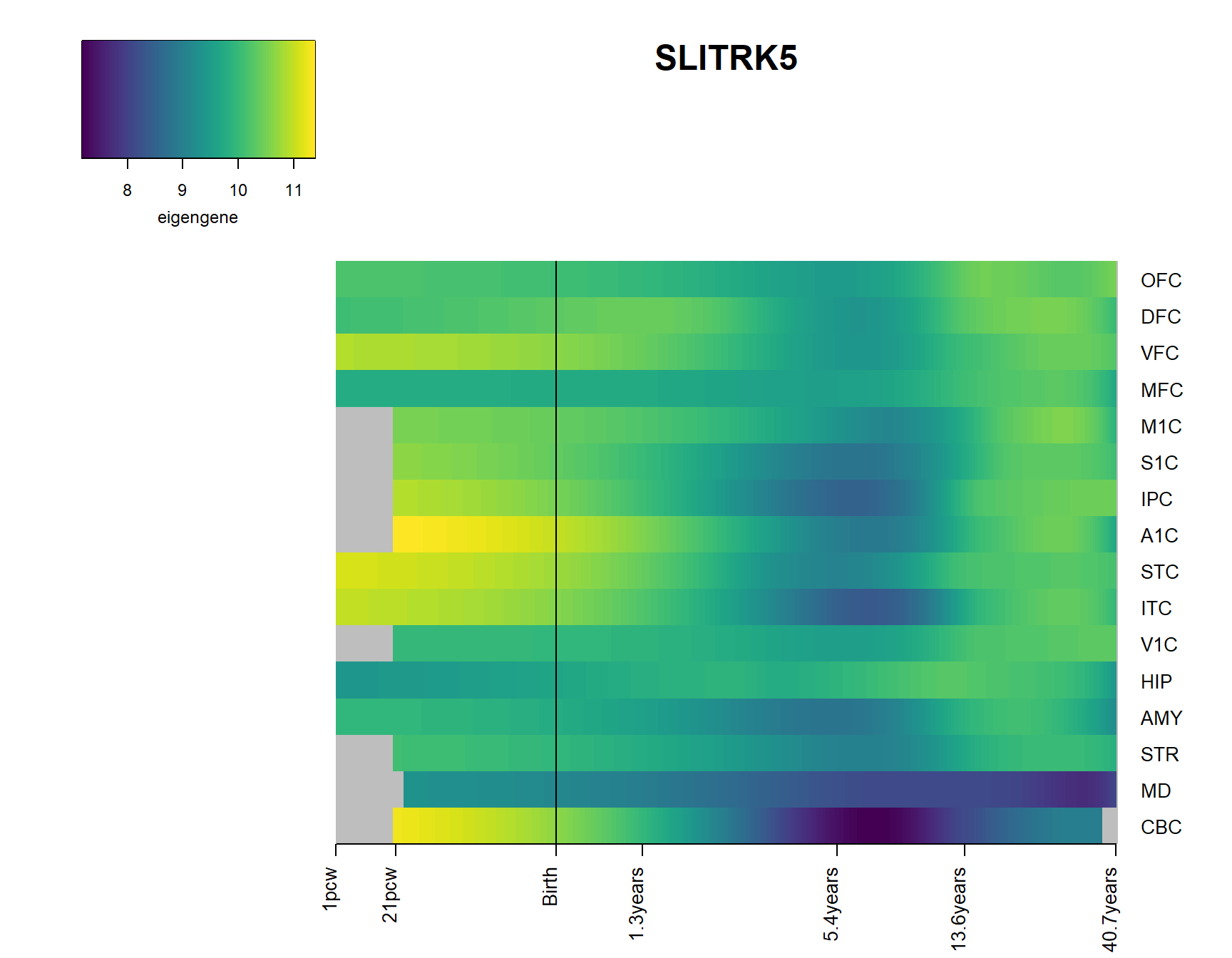
for(Module in Modsig2){
brainheatmap(Module)
}
pdf(paste0(Home, "/output/Brain_Module_Heatmap.pdf"))
brainheatmap_gene("SLITRK5")
for(Module in Modsig2){
brainheatmap(Module)
}
dev.off()png
2 Risk Factor Mediation Analysis
Risk factor loading and correlation plots
dropfact=c("site", "0", "group")
modelFact=strsplit(as.character(design(dds_filt))[2], " \\+ ")[[1]]
Patdata=as.data.frame(colData(dds_filt))
load(paste0(Home, "/output/envFact.RData"))
envFact=envFact[!envFact %in% dropfact]
modelFact=modelFact[!modelFact %in% dropfact]
EpiMarker = c()
# TopHit
Patdata$Epi_TopHit=log2_cpm[base::which.min(results_Deseq$pvalue),]
# 1PC of all diff met
tmp=glmpca(log2_cpm[base::which(results_Deseq$pvalue<=thresholdp),], 1)
Patdata$Epi_all= tmp$factors$dim1
EpiMarker = c(EpiMarker, "Epi_TopHit", "Epi_all")
#Brain Modules
Epitestset=GL_all[Modsig]
for(n in names(Epitestset)){
index=gettaglistforgenelist(genelist = Epitestset[[n]], dds_filt)
index = base::intersect(index, base::which(results_Deseq$pvalue<=thresholdp))
# get eigenvalue
epiname=paste0("Epi_",n)
tmp=glmpca(log2_cpm[index,], 1)
Patdata[,epiname]= tmp$factors$dim1
EpiMarker = c(EpiMarker, epiname)
}
cormat = cor(apply(Patdata[,c("group", envFact, modelFact, EpiMarker)] %>% mutate_all(as.numeric), 2, minmax_scaling),
use = "pairwise.complete.obs")
par(mfrow=c(1,2))
corrplot(cormat, main="correlations")
corrplot(cormat, order = "hclust", main="correlations ordered")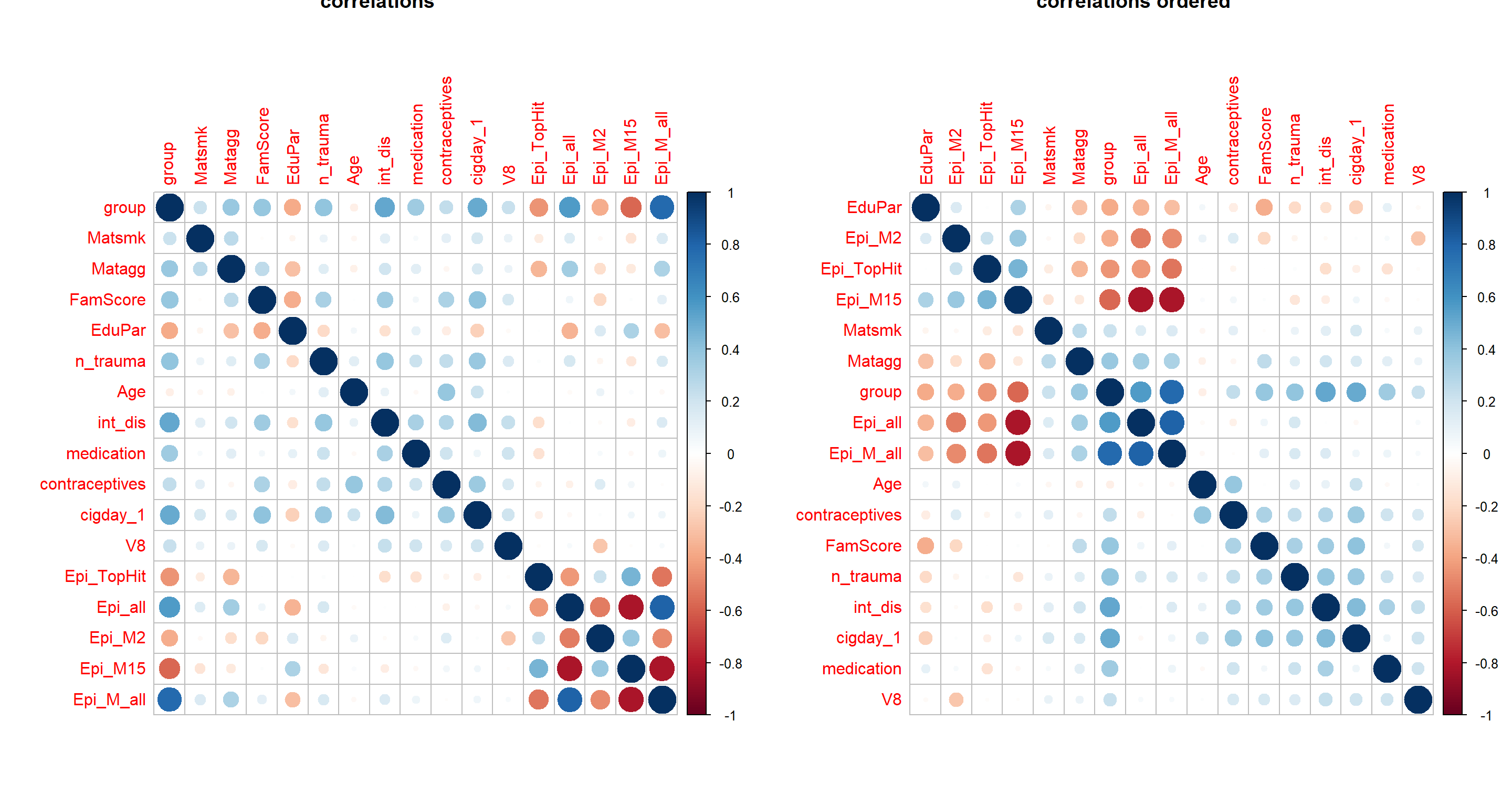
SEM analysis
fullmodEnv=paste(unique(envFact,modelFact), sep = "+", collapse = "+")
Dataset = Patdata[,c("group", envFact, modelFact,EpiMarker)]
model = "
Epi~0+a*Matsmk+b*Matagg+c*FamScore+d*EduPar+e*n_trauma+Age+int_dis+medication+contraceptives+cigday_1+V8
group~f*Matsmk+g*Matagg+h*FamScore+i*EduPar+j*n_trauma+Age+int_dis+medication+contraceptives+cigday_1+V8+z*Epi
#direct
directMatsmk := f
directMatagg := g
directFamScore := h
directEduPar := i
directn_trauma := j
#indirect
EpiMatsmk := a*z
EpiMatagg := b*z
EpiFamScore := c*z
EpiEduPar := d*z
Epin_trauma := e*z
total := f + g + h + i + j + (a*z)+(b*z)+(c*z)+(d*z)+(e*z)
"
Netlist = list()
nothing = function(x){return(x)}
for (marker in EpiMarker) {
Dataset$Epi = Dataset[,marker]
Datasetscaled = Dataset %>% mutate_if(is.numeric, minmax_scaling)
Datasetscaled = Datasetscaled %>% mutate_if(is.factor,ordered)
fit<-lavaan(model,data=Datasetscaled, estimator="DWLS")
sink(paste0(Home,"/output/SEM_summary_group",marker,".txt"))
summary(fit)
print(fitMeasures(fit))
print(parameterEstimates(fit))
sink()
cat("############################\n")
cat("############################\n")
cat(marker, "\n")
cat("############################\n")
cat("############################\n")
cat("##Mediation Model ##\n")
summary(fit)
cat("\n")
#print(fitMeasures(fit))
cat("\n")
#print(parameterEstimates(fit))
cat("\n")
#SOURCE FOR PLOT https://stackoverflow.com/questions/51270032/how-can-i-display-only-significant-path-lines-on-a-path-diagram-r-lavaan-sem
restab=lavaan::parameterEstimates(fit) %>% dplyr::filter(!is.na(pvalue)) %>%
arrange(desc(pvalue)) %>% mutate_if("is.numeric","round",3) %>%
dplyr::select(-ci.lower,-ci.upper,-z)
pvalue_cutoff <- 0.05
obj <- semPlot:::semPlotModel(fit)
original_Pars <- obj@Pars
print(original_Pars)
check_Pars <- obj@Pars %>% dplyr:::filter(!(edge %in% c("int","<->") | lhs == rhs)) # this is the list of parameter to sift thru
keep_Pars <- obj@Pars %>% dplyr:::filter(edge %in% c("int","<->") | lhs == rhs) # this is the list of parameter to keep asis
test_against <- lavaan::parameterEstimates(fit) %>% dplyr::filter(pvalue < pvalue_cutoff, rhs != lhs)
# for some reason, the rhs and lhs are reversed in the standardizedSolution() output, for some of the values
# I'll have to reverse it myself, and test against both orders
test_against_rev <- test_against %>% dplyr::rename(rhs2 = lhs, lhs = rhs) %>% dplyr::rename(rhs = rhs2)
checked_Pars <-
check_Pars %>% semi_join(test_against, by = c("lhs", "rhs")) %>% bind_rows(
check_Pars %>% semi_join(test_against_rev, by = c("lhs", "rhs"))
)
obj@Pars <- keep_Pars %>% bind_rows(checked_Pars) %>%
mutate_if("is.numeric","round",3) %>%
mutate_at(c("lhs","rhs"),~gsub("Epi", marker,.))
obj@Vars = obj@Vars %>% mutate_at(c("name"),~gsub("Epi", marker,.))
DF = obj@Pars
DF = DF[DF$lhs!=DF$rhs,]
DF = DF[abs(DF$est)>0.1,]
DF = DF[DF$edge == "~>",] # only include directly modelled effects in figure
nodes <- data.frame(id=obj@Vars$name, label = obj@Vars$name)
nodes$color<-Dark8[8]
nodes$color[nodes$label == "group"] = Dark8[3]
nodes$color[nodes$label == marker] = Dark8[4]
nodes$color[nodes$label %in% envFact] = Dark8[5]
if(nrow(DF)>0){
edges <- data.frame(from = DF$lhs,
to = DF$rhs,
width=abs(DF$est),
arrows ="to")
edges$dashes = F
edges$label = DF$est
edges$color=c("firebrick", "forestgreen")[1:2][factor(sign(DF$est), levels=c(-1,0,1),labels=c(1,2,2))]
edges$width=2
}
else {edges = data.frame(from=NULL, to = NULL)}
cexlab = 18
plotnet<- visNetwork(nodes, edges,
main=list(text=marker,
style="font-family:arial;font-size:20px;text-align:center"),
submain=list(text="significant paths",
style="font-family:arial;text-align:center")) %>%
visEdges(arrows =list(to = list(enabled = TRUE, scaleFactor = 0.7)),
font=list(size=cexlab, style="font-family:arial;text-align:center")) %>%
visNodes(font=list(size=cexlab, style="font-family:arial;text-align:center")) %>%
visPhysics(enabled = T, solver = "forceAtlas2Based")
Netlist[[marker]] = plotnet
htmlfile = paste0(Home,"/output/SEMplot_",marker,".html")
visSave(plotnet, htmlfile)
webshot(paste0(Home,"/output/SEMplot_",marker,".html"), zoom = 1,
file = paste0(Home,"/output/SEMplot_",marker,".png"))
}Warning in lav_data_full(data = data, group = group, cluster = cluster, :
lavaan WARNING: exogenous variable(s) declared as ordered in data: Matsmk Matagg
int_dis medication contraceptivesWarning in lav_samplestats_step2(UNI = FIT, wt = wt, ov.names = ov.names, :
lavaan WARNING: correlation between variables group and Epi is (nearly) 1.0Warning in lav_object_post_check(object): lavaan WARNING: some estimated ov
variances are negative############################
############################
Epi_TopHit
############################
############################
##Mediation Model ##
lavaan 0.6-7 ended normally after 108 iterations
Estimator DWLS
Optimization method NLMINB
Number of free parameters 23
Used Total
Number of observations 80 99
Model Test User Model:
Test statistic 152.293
Degrees of freedom 3
P-value (Chi-square) 0.000
Parameter Estimates:
Standard errors Standard
Information Expected
Information saturated (h1) model Unstructured
Regressions:
Estimate Std.Err z-value P(>|z|)
Epi ~
Matsmk (a) -0.033 0.050 -0.657 0.511
Matagg (b) -0.014 0.072 -0.202 0.840
FamScore (c) 0.056 0.076 0.739 0.460
EduPar (d) -0.038 0.108 -0.358 0.721
n_trauma (e) 0.085 0.111 0.767 0.443
Age -0.090 0.097 -0.929 0.353
int_dis -0.074 0.057 -1.310 0.190
medication -0.044 0.059 -0.759 0.448
contrcptvs -0.017 0.049 -0.341 0.733
cigday_1 -0.111 0.136 -0.815 0.415
V8 -0.089 0.568 -0.156 0.876
group ~
Matsmk (f) 0.194 1.160 0.167 0.867
Matagg (g) 1.502 2.502 0.600 0.548
FamScore (h) 0.182 2.108 0.086 0.931
EduPar (i) -3.511 2.254 -1.558 0.119
n_trauma (j) 2.563 1.452 1.766 0.077
Age -3.056 2.522 -1.211 0.226
int_dis 1.143 0.829 1.379 0.168
medication 1.079 0.873 1.235 0.217
contrcptvs 0.108 0.803 0.135 0.893
cigday_1 10.186 7.209 1.413 0.158
V8 13.100 16.931 0.774 0.439
Epi (z) -1.921 0.014 -133.612 0.000
Intercepts:
Estimate Std.Err z-value P(>|z|)
.Epi 0.000
.group 0.000
Thresholds:
Estimate Std.Err z-value P(>|z|)
group|t1 10.125
Variances:
Estimate Std.Err z-value P(>|z|)
.Epi 1.000
.group -2.692
Scales y*:
Estimate Std.Err z-value P(>|z|)
group 1.000
Defined Parameters:
Estimate Std.Err z-value P(>|z|)
directMatsmk 0.194 1.160 0.167 0.867
directMatagg 1.502 2.502 0.600 0.548
directFamScore 0.182 2.108 0.086 0.931
directEduPar -3.511 2.254 -1.558 0.119
directn_trauma 2.563 1.452 1.766 0.077
EpiMatsmk 0.063 0.095 0.657 0.511
EpiMatagg 0.028 0.138 0.202 0.840
EpiFamScore -0.108 0.147 -0.739 0.460
EpiEduPar 0.074 0.207 0.358 0.721
Epin_trauma -0.163 0.213 -0.766 0.443
total 0.823 4.371 0.188 0.851
label lhs edge rhs est std group
1 int Epi 0.000000e+00 0.0000000000
2 a Matsmk ~> Epi -3.256589e-02 -0.0143904486
3 b Matagg ~> Epi -1.446988e-02 -0.0043596534
4 c FamScore ~> Epi 5.638498e-02 0.0204664407
5 d EduPar ~> Epi -3.844427e-02 -0.0088987514
6 e n_trauma ~> Epi 8.486733e-02 0.0191918495
7 Age ~> Epi -9.011774e-02 -0.0195992540
8 int_dis ~> Epi -7.447596e-02 -0.0342761092
9 medication ~> Epi -4.445645e-02 -0.0169647132
10 contraceptives ~> Epi -1.677936e-02 -0.0077223731
11 cigday_1 ~> Epi -1.109172e-01 -0.0274559411
12 V8 ~> Epi -8.888122e-02 -0.0060402510
13 f Matsmk ~> group 1.936833e-01 0.0213188597
14 g Matagg ~> group 1.502114e+00 0.1127328109
15 h FamScore ~> group 1.819577e-01 0.0164516644
16 i EduPar ~> group -3.511101e+00 -0.2024423080
17 j n_trauma ~> group 2.563273e+00 0.1443881569
18 Age ~> group -3.055589e+00 -0.1655330451
19 int_dis ~> group 1.143258e+00 0.1310630086
20 medication ~> group 1.078602e+00 0.1025259011
21 contraceptives ~> group 1.080677e-01 0.0123888743
22 cigday_1 ~> group 1.018628e+01 0.6280782404
23 V8 ~> group 1.310041e+01 0.2217637315
24 z Epi ~> group -1.921485e+00 -0.4786273427
26 Epi <-> Epi 1.000000e+00 0.9960212963
27 group <-> group -2.692105e+00 -0.1663725191
28 Matsmk <-> Matsmk 1.960443e-01 1.0000000000
29 Matsmk <-> Matagg 4.936709e-02 0.3693241433
30 Matsmk <-> FamScore -9.177215e-03 -0.0569887592
31 Matsmk <-> EduPar -5.564346e-03 -0.0541843459
32 Matsmk <-> n_trauma 7.459313e-03 0.0743497863
33 Matsmk <-> Age -3.217300e-03 -0.0333441329
34 Matsmk <-> int_dis 2.151899e-02 0.1053910232
35 Matsmk <-> medication 4.113924e-03 0.0242997446
36 Matsmk <-> contraceptives 2.151899e-02 0.1053910232
37 Matsmk <-> cigday_1 1.693829e-02 0.1542372547
38 Matsmk <-> V8 2.928139e-03 0.0971189320
39 Matagg <-> Matagg 9.113924e-02 1.0000000000
40 Matagg <-> FamScore 3.417722e-02 0.3112715087
41 Matagg <-> EduPar -1.656118e-02 -0.2365241196
42 Matagg <-> n_trauma 7.233273e-03 0.1057402114
43 Matagg <-> Age 3.118694e-04 0.0047405101
44 Matagg <-> int_dis 3.291139e-02 0.2364027144
45 Matagg <-> medication 7.594937e-03 0.0657951695
46 Matagg <-> contraceptives 7.594937e-03 0.0545544726
47 Matagg <-> cigday_1 1.018987e-02 0.1360858260
48 Matagg <-> V8 8.272067e-04 0.0402393217
49 FamScore <-> FamScore 1.322785e-01 1.0000000000
50 FamScore <-> EduPar -2.948312e-02 -0.3495149022
51 FamScore <-> n_trauma 2.667269e-02 0.3236534989
52 FamScore <-> Age 3.636947e-03 0.0458878230
53 FamScore <-> int_dis 6.455696e-02 0.3849084009
54 FamScore <-> medication 4.430380e-03 0.0318580293
55 FamScore <-> contraceptives 5.822785e-02 0.3471722832
56 FamScore <-> cigday_1 4.381329e-02 0.4856887960
57 FamScore <-> V8 7.814844e-04 0.0315547719
58 EduPar <-> EduPar 5.379307e-02 1.0000000000
59 EduPar <-> n_trauma -8.024412e-03 -0.1526891136
60 EduPar <-> Age 2.762108e-03 0.0546490350
61 EduPar <-> int_dis -1.909283e-02 -0.1785114035
62 EduPar <-> medication 1.017932e-02 0.1147832062
63 EduPar <-> contraceptives -1.329114e-02 -0.1242676068
64 EduPar <-> cigday_1 -1.493803e-02 -0.2596730517
65 EduPar <-> V8 -8.860887e-06 -0.0005610523
66 n_trauma <-> n_trauma 5.134332e-02 1.0000000000
67 n_trauma <-> Age 1.582278e-03 0.0320439451
68 n_trauma <-> int_dis 4.159132e-02 0.3980335009
69 n_trauma <-> medication 1.763110e-02 0.2034979577
70 n_trauma <-> contraceptives 1.808318e-02 0.1730580439
71 n_trauma <-> cigday_1 2.128165e-02 0.3786692420
72 n_trauma <-> V8 -4.694340e-04 -0.0304243917
73 Age <-> Age 4.748866e-02 1.0000000000
74 Age <-> int_dis 8.090259e-03 0.0805056484
75 Age <-> medication -1.655660e-03 -0.0198700345
76 Age <-> contraceptives 3.524124e-02 0.3506833348
77 Age <-> cigday_1 8.542355e-03 0.1580445206
78 Age <-> V8 -1.333633e-03 -0.0898732659
79 int_dis <-> int_dis 2.126582e-01 1.0000000000
80 int_dis <-> medication 6.075949e-02 0.3445843938
81 int_dis <-> contraceptives 6.075949e-02 0.2857142857
82 int_dis <-> cigday_1 4.449367e-02 0.3890038953
83 int_dis <-> V8 5.645344e-03 0.1797788722
84 medication <-> medication 1.462025e-01 1.0000000000
85 medication <-> contraceptives 3.544304e-02 0.2010075631
86 medication <-> cigday_1 3.275316e-03 0.0345360471
87 medication <-> V8 2.084232e-03 0.0800493604
88 contraceptives <-> contraceptives 2.126582e-01 1.0000000000
89 contraceptives <-> cigday_1 4.892405e-02 0.4277382803
90 contraceptives <-> V8 2.688833e-03 0.0856272484
91 cigday_1 <-> cigday_1 6.151859e-02 1.0000000000
92 cigday_1 <-> V8 1.509276e-03 0.0893623867
93 V8 <-> V8 4.636832e-03 1.0000000000
94 group <-> group 1.000000e+00 1.0000000000
95 int group 0.000000e+00 0.0000000000
96 int Matsmk 1.262500e+00 2.8513745747
97 int Matagg 1.100000e+00 3.6436779343
98 int FamScore 2.250000e-01 0.6186398880
99 int EduPar 6.062500e-01 2.6138976225
100 int n_trauma 1.964286e-01 0.8668873691
101 int Age 5.621377e-01 2.5795724974
102 int int_dis 1.300000e+00 2.8190466136
103 int medication 1.175000e+00 3.0729848569
104 int contraceptives 1.300000e+00 2.8190466136
105 int cigday_1 1.243750e-01 0.5014526157
106 int V8 5.286908e-01 7.7640983340
fixed par
1 TRUE 0
2 FALSE 1
3 FALSE 2
4 FALSE 3
5 FALSE 4
6 FALSE 5
7 FALSE 6
8 FALSE 7
9 FALSE 8
10 FALSE 9
11 FALSE 10
12 FALSE 11
13 FALSE 12
14 FALSE 13
15 FALSE 14
16 FALSE 15
17 FALSE 16
18 FALSE 17
19 FALSE 18
20 FALSE 19
21 FALSE 20
22 FALSE 21
23 FALSE 22
24 FALSE 23
26 TRUE 0
27 TRUE 0
28 TRUE 0
29 TRUE 0
30 TRUE 0
31 TRUE 0
32 TRUE 0
33 TRUE 0
34 TRUE 0
35 TRUE 0
36 TRUE 0
37 TRUE 0
38 TRUE 0
39 TRUE 0
40 TRUE 0
41 TRUE 0
42 TRUE 0
43 TRUE 0
44 TRUE 0
45 TRUE 0
46 TRUE 0
47 TRUE 0
48 TRUE 0
49 TRUE 0
50 TRUE 0
51 TRUE 0
52 TRUE 0
53 TRUE 0
54 TRUE 0
55 TRUE 0
56 TRUE 0
57 TRUE 0
58 TRUE 0
59 TRUE 0
60 TRUE 0
61 TRUE 0
62 TRUE 0
63 TRUE 0
64 TRUE 0
65 TRUE 0
66 TRUE 0
67 TRUE 0
68 TRUE 0
69 TRUE 0
70 TRUE 0
71 TRUE 0
72 TRUE 0
73 TRUE 0
74 TRUE 0
75 TRUE 0
76 TRUE 0
77 TRUE 0
78 TRUE 0
79 TRUE 0
80 TRUE 0
81 TRUE 0
82 TRUE 0
83 TRUE 0
84 TRUE 0
85 TRUE 0
86 TRUE 0
87 TRUE 0
88 TRUE 0
89 TRUE 0
90 TRUE 0
91 TRUE 0
92 TRUE 0
93 TRUE 0
94 TRUE 0
95 TRUE 0
96 TRUE 0
97 TRUE 0
98 TRUE 0
99 TRUE 0
100 TRUE 0
101 TRUE 0
102 TRUE 0
103 TRUE 0
104 TRUE 0
105 TRUE 0
106 TRUE 0Warning in lav_data_full(data = data, group = group, cluster = cluster, :
lavaan WARNING: exogenous variable(s) declared as ordered in data: Matsmk Matagg
int_dis medication contraceptivesWarning in lav_samplestats_step2(UNI = FIT, wt = wt, ov.names = ov.names, :
lavaan WARNING: correlation between variables group and Epi is (nearly) 1.0Warning in lav_object_post_check(object): lavaan WARNING: some estimated ov
variances are negative############################
############################
Epi_all
############################
############################
##Mediation Model ##
lavaan 0.6-7 ended normally after 114 iterations
Estimator DWLS
Optimization method NLMINB
Number of free parameters 23
Used Total
Number of observations 80 99
Model Test User Model:
Test statistic 26.894
Degrees of freedom 3
P-value (Chi-square) 0.000
Parameter Estimates:
Standard errors Standard
Information Expected
Information saturated (h1) model Unstructured
Regressions:
Estimate Std.Err z-value P(>|z|)
Epi ~
Matsmk (a) 0.014 0.025 0.573 0.567
Matagg (b) 0.023 0.038 0.590 0.555
FamScore (c) -0.037 0.042 -0.871 0.384
EduPar (d) -0.090 0.060 -1.514 0.130
n_trauma (e) 0.025 0.048 0.523 0.601
Age 0.034 0.064 0.534 0.593
int_dis 0.023 0.024 0.934 0.350
medication 0.007 0.030 0.248 0.804
contrcptvs -0.016 0.027 -0.583 0.560
cigday_1 0.022 0.052 0.425 0.671
V8 0.060 0.306 0.197 0.844
group ~
Matsmk (f) 0.236 1.156 0.204 0.838
Matagg (g) 1.498 2.499 0.600 0.549
FamScore (h) 0.125 2.104 0.059 0.953
EduPar (i) -3.311 2.246 -1.474 0.140
n_trauma (j) 2.365 1.437 1.645 0.100
Age -2.930 2.517 -1.164 0.244
int_dis 1.254 0.822 1.525 0.127
medication 1.154 0.867 1.331 0.183
contrcptvs 0.162 0.798 0.203 0.839
cigday_1 10.369 7.205 1.439 0.150
V8 13.187 16.901 0.780 0.435
Epi (z) 1.393 0.134 10.435 0.000
Intercepts:
Estimate Std.Err z-value P(>|z|)
.Epi 0.000
.group 0.000
Thresholds:
Estimate Std.Err z-value P(>|z|)
group|t1 10.125
Variances:
Estimate Std.Err z-value P(>|z|)
.Epi 1.000
.group -0.942
Scales y*:
Estimate Std.Err z-value P(>|z|)
group 1.000
Defined Parameters:
Estimate Std.Err z-value P(>|z|)
directMatsmk 0.236 1.156 0.204 0.838
directMatagg 1.498 2.499 0.600 0.549
directFamScore 0.125 2.104 0.059 0.953
directEduPar -3.311 2.246 -1.474 0.140
directn_trauma 2.365 1.437 1.645 0.100
EpiMatsmk 0.020 0.035 0.572 0.567
EpiMatagg 0.032 0.054 0.590 0.556
EpiFamScore -0.051 0.059 -0.868 0.385
EpiEduPar -0.126 0.084 -1.499 0.134
Epin_trauma 0.035 0.067 0.522 0.602
total 0.823 4.371 0.188 0.851
label lhs edge rhs est std group
1 int Epi 0.000000e+00 0.0000000000
2 a Matsmk ~> Epi 1.438673e-02 0.0063670252
3 b Matagg ~> Epi 2.267876e-02 0.0068433600
4 c FamScore ~> Epi -3.652791e-02 -0.0132790452
5 d EduPar ~> Epi -9.048835e-02 -0.0209774780
6 e n_trauma ~> Epi 2.508125e-02 0.0056805267
7 Age ~> Epi 3.417168e-02 0.0074431841
8 int_dis ~> Epi 2.287155e-02 0.0105422718
9 medication ~> Epi 7.364844e-03 0.0028147411
10 contraceptives ~> Epi -1.577718e-02 -0.0072722355
11 cigday_1 ~> Epi 2.200542e-02 0.0054554452
12 V8 ~> Epi 6.028764e-02 0.0041033295
13 f Matsmk ~> group 2.362105e-01 0.0259998623
14 g Matagg ~> group 1.498314e+00 0.1124475911
15 h FamScore ~> group 1.245177e-01 0.0112582385
16 i EduPar ~> group -3.311137e+00 -0.1909128197
17 j n_trauma ~> group 2.365251e+00 0.1332336696
18 Age ~> group -2.930048e+00 -0.1587319632
19 int_dis ~> group 1.254491e+00 0.1438147828
20 medication ~> group 1.153762e+00 0.1096701472
21 contraceptives ~> group 1.622942e-01 0.0186053939
22 cigday_1 ~> group 1.036875e+01 0.6393286187
23 V8 ~> group 1.318719e+01 0.2232327002
24 z Epi ~> group 1.393481e+00 0.3465760225
26 Epi <-> Epi 1.000000e+00 0.9990675738
27 group <-> group -9.417905e-01 -0.0582027933
28 Matsmk <-> Matsmk 1.960443e-01 1.0000000000
29 Matsmk <-> Matagg 4.936709e-02 0.3693241433
30 Matsmk <-> FamScore -9.177215e-03 -0.0569887592
31 Matsmk <-> EduPar -5.564346e-03 -0.0541843459
32 Matsmk <-> n_trauma 7.459313e-03 0.0743497863
33 Matsmk <-> Age -3.217300e-03 -0.0333441329
34 Matsmk <-> int_dis 2.151899e-02 0.1053910232
35 Matsmk <-> medication 4.113924e-03 0.0242997446
36 Matsmk <-> contraceptives 2.151899e-02 0.1053910232
37 Matsmk <-> cigday_1 1.693829e-02 0.1542372547
38 Matsmk <-> V8 2.928139e-03 0.0971189320
39 Matagg <-> Matagg 9.113924e-02 1.0000000000
40 Matagg <-> FamScore 3.417722e-02 0.3112715087
41 Matagg <-> EduPar -1.656118e-02 -0.2365241196
42 Matagg <-> n_trauma 7.233273e-03 0.1057402114
43 Matagg <-> Age 3.118694e-04 0.0047405101
44 Matagg <-> int_dis 3.291139e-02 0.2364027144
45 Matagg <-> medication 7.594937e-03 0.0657951695
46 Matagg <-> contraceptives 7.594937e-03 0.0545544726
47 Matagg <-> cigday_1 1.018987e-02 0.1360858260
48 Matagg <-> V8 8.272067e-04 0.0402393217
49 FamScore <-> FamScore 1.322785e-01 1.0000000000
50 FamScore <-> EduPar -2.948312e-02 -0.3495149022
51 FamScore <-> n_trauma 2.667269e-02 0.3236534989
52 FamScore <-> Age 3.636947e-03 0.0458878230
53 FamScore <-> int_dis 6.455696e-02 0.3849084009
54 FamScore <-> medication 4.430380e-03 0.0318580293
55 FamScore <-> contraceptives 5.822785e-02 0.3471722832
56 FamScore <-> cigday_1 4.381329e-02 0.4856887960
57 FamScore <-> V8 7.814844e-04 0.0315547719
58 EduPar <-> EduPar 5.379307e-02 1.0000000000
59 EduPar <-> n_trauma -8.024412e-03 -0.1526891136
60 EduPar <-> Age 2.762108e-03 0.0546490350
61 EduPar <-> int_dis -1.909283e-02 -0.1785114035
62 EduPar <-> medication 1.017932e-02 0.1147832062
63 EduPar <-> contraceptives -1.329114e-02 -0.1242676068
64 EduPar <-> cigday_1 -1.493803e-02 -0.2596730517
65 EduPar <-> V8 -8.860887e-06 -0.0005610523
66 n_trauma <-> n_trauma 5.134332e-02 1.0000000000
67 n_trauma <-> Age 1.582278e-03 0.0320439451
68 n_trauma <-> int_dis 4.159132e-02 0.3980335009
69 n_trauma <-> medication 1.763110e-02 0.2034979577
70 n_trauma <-> contraceptives 1.808318e-02 0.1730580439
71 n_trauma <-> cigday_1 2.128165e-02 0.3786692420
72 n_trauma <-> V8 -4.694340e-04 -0.0304243917
73 Age <-> Age 4.748866e-02 1.0000000000
74 Age <-> int_dis 8.090259e-03 0.0805056484
75 Age <-> medication -1.655660e-03 -0.0198700345
76 Age <-> contraceptives 3.524124e-02 0.3506833348
77 Age <-> cigday_1 8.542355e-03 0.1580445206
78 Age <-> V8 -1.333633e-03 -0.0898732659
79 int_dis <-> int_dis 2.126582e-01 1.0000000000
80 int_dis <-> medication 6.075949e-02 0.3445843938
81 int_dis <-> contraceptives 6.075949e-02 0.2857142857
82 int_dis <-> cigday_1 4.449367e-02 0.3890038953
83 int_dis <-> V8 5.645344e-03 0.1797788722
84 medication <-> medication 1.462025e-01 1.0000000000
85 medication <-> contraceptives 3.544304e-02 0.2010075631
86 medication <-> cigday_1 3.275316e-03 0.0345360471
87 medication <-> V8 2.084232e-03 0.0800493604
88 contraceptives <-> contraceptives 2.126582e-01 1.0000000000
89 contraceptives <-> cigday_1 4.892405e-02 0.4277382803
90 contraceptives <-> V8 2.688833e-03 0.0856272484
91 cigday_1 <-> cigday_1 6.151859e-02 1.0000000000
92 cigday_1 <-> V8 1.509276e-03 0.0893623867
93 V8 <-> V8 4.636832e-03 1.0000000000
94 group <-> group 1.000000e+00 1.0000000000
95 int group 0.000000e+00 0.0000000000
96 int Matsmk 1.262500e+00 2.8513745747
97 int Matagg 1.100000e+00 3.6436779343
98 int FamScore 2.250000e-01 0.6186398880
99 int EduPar 6.062500e-01 2.6138976225
100 int n_trauma 1.964286e-01 0.8668873691
101 int Age 5.621377e-01 2.5795724974
102 int int_dis 1.300000e+00 2.8190466136
103 int medication 1.175000e+00 3.0729848569
104 int contraceptives 1.300000e+00 2.8190466136
105 int cigday_1 1.243750e-01 0.5014526157
106 int V8 5.286908e-01 7.7640983340
fixed par
1 TRUE 0
2 FALSE 1
3 FALSE 2
4 FALSE 3
5 FALSE 4
6 FALSE 5
7 FALSE 6
8 FALSE 7
9 FALSE 8
10 FALSE 9
11 FALSE 10
12 FALSE 11
13 FALSE 12
14 FALSE 13
15 FALSE 14
16 FALSE 15
17 FALSE 16
18 FALSE 17
19 FALSE 18
20 FALSE 19
21 FALSE 20
22 FALSE 21
23 FALSE 22
24 FALSE 23
26 TRUE 0
27 TRUE 0
28 TRUE 0
29 TRUE 0
30 TRUE 0
31 TRUE 0
32 TRUE 0
33 TRUE 0
34 TRUE 0
35 TRUE 0
36 TRUE 0
37 TRUE 0
38 TRUE 0
39 TRUE 0
40 TRUE 0
41 TRUE 0
42 TRUE 0
43 TRUE 0
44 TRUE 0
45 TRUE 0
46 TRUE 0
47 TRUE 0
48 TRUE 0
49 TRUE 0
50 TRUE 0
51 TRUE 0
52 TRUE 0
53 TRUE 0
54 TRUE 0
55 TRUE 0
56 TRUE 0
57 TRUE 0
58 TRUE 0
59 TRUE 0
60 TRUE 0
61 TRUE 0
62 TRUE 0
63 TRUE 0
64 TRUE 0
65 TRUE 0
66 TRUE 0
67 TRUE 0
68 TRUE 0
69 TRUE 0
70 TRUE 0
71 TRUE 0
72 TRUE 0
73 TRUE 0
74 TRUE 0
75 TRUE 0
76 TRUE 0
77 TRUE 0
78 TRUE 0
79 TRUE 0
80 TRUE 0
81 TRUE 0
82 TRUE 0
83 TRUE 0
84 TRUE 0
85 TRUE 0
86 TRUE 0
87 TRUE 0
88 TRUE 0
89 TRUE 0
90 TRUE 0
91 TRUE 0
92 TRUE 0
93 TRUE 0
94 TRUE 0
95 TRUE 0
96 TRUE 0
97 TRUE 0
98 TRUE 0
99 TRUE 0
100 TRUE 0
101 TRUE 0
102 TRUE 0
103 TRUE 0
104 TRUE 0
105 TRUE 0
106 TRUE 0Warning in lav_data_full(data = data, group = group, cluster = cluster, :
lavaan WARNING: exogenous variable(s) declared as ordered in data: Matsmk Matagg
int_dis medication contraceptives
Warning in lav_data_full(data = data, group = group, cluster = cluster, : lavaan
WARNING: some estimated ov variances are negative############################
############################
Epi_M2
############################
############################
##Mediation Model ##
lavaan 0.6-7 ended normally after 108 iterations
Estimator DWLS
Optimization method NLMINB
Number of free parameters 23
Used Total
Number of observations 80 99
Model Test User Model:
Test statistic 243.503
Degrees of freedom 3
P-value (Chi-square) 0.000
Parameter Estimates:
Standard errors Standard
Information Expected
Information saturated (h1) model Unstructured
Regressions:
Estimate Std.Err z-value P(>|z|)
Epi ~
Matsmk (a) 0.009 0.042 0.217 0.829
Matagg (b) -0.017 0.064 -0.261 0.794
FamScore (c) -0.054 0.063 -0.853 0.393
EduPar (d) -0.010 0.092 -0.109 0.913
n_trauma (e) 0.018 0.096 0.188 0.851
Age -0.042 0.101 -0.416 0.677
int_dis -0.010 0.038 -0.255 0.799
medication 0.018 0.039 0.453 0.651
contrcptvs 0.074 0.053 1.390 0.165
cigday_1 -0.058 0.091 -0.641 0.522
V8 -0.522 0.239 -2.187 0.029
group ~
Matsmk (f) 0.308 1.180 0.261 0.794
Matagg (g) 1.435 2.525 0.568 0.570
FamScore (h) -0.234 2.134 -0.110 0.913
EduPar (i) -3.495 2.306 -1.516 0.130
n_trauma (j) 2.505 1.539 1.627 0.104
Age -3.123 2.581 -1.210 0.226
int_dis 1.231 0.850 1.449 0.147
medication 1.267 0.895 1.415 0.157
contrcptvs 0.566 0.854 0.663 0.508
cigday_1 10.065 7.223 1.393 0.163
V8 10.274 16.951 0.606 0.544
Epi (z) -5.741 0.009 -669.813 0.000
Intercepts:
Estimate Std.Err z-value P(>|z|)
.Epi 0.000
.group 0.000
Thresholds:
Estimate Std.Err z-value P(>|z|)
group|t1 10.125
Variances:
Estimate Std.Err z-value P(>|z|)
.Epi 1.000
.group -31.957
Scales y*:
Estimate Std.Err z-value P(>|z|)
group 1.000
Defined Parameters:
Estimate Std.Err z-value P(>|z|)
directMatsmk 0.308 1.180 0.261 0.794
directMatagg 1.435 2.525 0.568 0.570
directFamScore -0.234 2.134 -0.110 0.913
directEduPar -3.495 2.306 -1.516 0.130
directn_trauma 2.505 1.539 1.627 0.104
EpiMatsmk -0.052 0.239 -0.217 0.829
EpiMatagg 0.095 0.365 0.261 0.794
EpiFamScore 0.308 0.361 0.853 0.393
EpiEduPar 0.058 0.526 0.109 0.913
Epin_trauma -0.104 0.554 -0.188 0.851
total 0.823 4.371 0.188 0.851
label lhs edge rhs est std group
1 int Epi 0.000000e+00 0.0000000000
2 a Matsmk ~> Epi 9.021717e-03 0.0039897063
3 b Matagg ~> Epi -1.656139e-02 -0.0049937195
4 c FamScore ~> Epi -5.363066e-02 -0.0194819461
5 d EduPar ~> Epi -1.003054e-02 -0.0023236029
6 e n_trauma ~> Epi 1.817152e-02 0.0041125181
7 Age ~> Epi -4.183870e-02 -0.0091064217
8 int_dis ~> Epi -9.609870e-03 -0.0044262219
9 medication ~> Epi 1.786833e-02 0.0068239536
10 contraceptives ~> Epi 7.415666e-02 0.0341559075
11 cigday_1 ~> Epi -5.823695e-02 -0.0144270114
12 V8 ~> Epi -5.220008e-01 -0.0355023001
13 f Matsmk ~> group 3.080500e-01 0.0339073236
14 g Matagg ~> group 1.434825e+00 0.1076829255
15 h FamScore ~> group -2.342742e-01 -0.0211818619
16 i EduPar ~> group -3.494814e+00 -0.2015033912
17 j n_trauma ~> group 2.504516e+00 0.1410785378
18 Age ~> group -3.122626e+00 -0.1691648440
19 int_dis ~> group 1.231194e+00 0.1411440750
20 medication ~> group 1.266603e+00 0.1203963286
21 contraceptives ~> group 5.660290e-01 0.0648895675
22 cigday_1 ~> group 1.006508e+01 0.6206054463
23 V8 ~> group 1.027449e+01 0.1739266838
24 z Epi ~> group -5.740817e+00 -1.4288749907
26 Epi <-> Epi 1.000000e+00 0.9975833547
27 group <-> group -3.195698e+01 -1.9749494619
28 Matsmk <-> Matsmk 1.960443e-01 1.0000000000
29 Matsmk <-> Matagg 4.936709e-02 0.3693241433
30 Matsmk <-> FamScore -9.177215e-03 -0.0569887592
31 Matsmk <-> EduPar -5.564346e-03 -0.0541843459
32 Matsmk <-> n_trauma 7.459313e-03 0.0743497863
33 Matsmk <-> Age -3.217300e-03 -0.0333441329
34 Matsmk <-> int_dis 2.151899e-02 0.1053910232
35 Matsmk <-> medication 4.113924e-03 0.0242997446
36 Matsmk <-> contraceptives 2.151899e-02 0.1053910232
37 Matsmk <-> cigday_1 1.693829e-02 0.1542372547
38 Matsmk <-> V8 2.928139e-03 0.0971189320
39 Matagg <-> Matagg 9.113924e-02 1.0000000000
40 Matagg <-> FamScore 3.417722e-02 0.3112715087
41 Matagg <-> EduPar -1.656118e-02 -0.2365241196
42 Matagg <-> n_trauma 7.233273e-03 0.1057402114
43 Matagg <-> Age 3.118694e-04 0.0047405101
44 Matagg <-> int_dis 3.291139e-02 0.2364027144
45 Matagg <-> medication 7.594937e-03 0.0657951695
46 Matagg <-> contraceptives 7.594937e-03 0.0545544726
47 Matagg <-> cigday_1 1.018987e-02 0.1360858260
48 Matagg <-> V8 8.272067e-04 0.0402393217
49 FamScore <-> FamScore 1.322785e-01 1.0000000000
50 FamScore <-> EduPar -2.948312e-02 -0.3495149022
51 FamScore <-> n_trauma 2.667269e-02 0.3236534989
52 FamScore <-> Age 3.636947e-03 0.0458878230
53 FamScore <-> int_dis 6.455696e-02 0.3849084009
54 FamScore <-> medication 4.430380e-03 0.0318580293
55 FamScore <-> contraceptives 5.822785e-02 0.3471722832
56 FamScore <-> cigday_1 4.381329e-02 0.4856887960
57 FamScore <-> V8 7.814844e-04 0.0315547719
58 EduPar <-> EduPar 5.379307e-02 1.0000000000
59 EduPar <-> n_trauma -8.024412e-03 -0.1526891136
60 EduPar <-> Age 2.762108e-03 0.0546490350
61 EduPar <-> int_dis -1.909283e-02 -0.1785114035
62 EduPar <-> medication 1.017932e-02 0.1147832062
63 EduPar <-> contraceptives -1.329114e-02 -0.1242676068
64 EduPar <-> cigday_1 -1.493803e-02 -0.2596730517
65 EduPar <-> V8 -8.860887e-06 -0.0005610523
66 n_trauma <-> n_trauma 5.134332e-02 1.0000000000
67 n_trauma <-> Age 1.582278e-03 0.0320439451
68 n_trauma <-> int_dis 4.159132e-02 0.3980335009
69 n_trauma <-> medication 1.763110e-02 0.2034979577
70 n_trauma <-> contraceptives 1.808318e-02 0.1730580439
71 n_trauma <-> cigday_1 2.128165e-02 0.3786692420
72 n_trauma <-> V8 -4.694340e-04 -0.0304243917
73 Age <-> Age 4.748866e-02 1.0000000000
74 Age <-> int_dis 8.090259e-03 0.0805056484
75 Age <-> medication -1.655660e-03 -0.0198700345
76 Age <-> contraceptives 3.524124e-02 0.3506833348
77 Age <-> cigday_1 8.542355e-03 0.1580445206
78 Age <-> V8 -1.333633e-03 -0.0898732659
79 int_dis <-> int_dis 2.126582e-01 1.0000000000
80 int_dis <-> medication 6.075949e-02 0.3445843938
81 int_dis <-> contraceptives 6.075949e-02 0.2857142857
82 int_dis <-> cigday_1 4.449367e-02 0.3890038953
83 int_dis <-> V8 5.645344e-03 0.1797788722
84 medication <-> medication 1.462025e-01 1.0000000000
85 medication <-> contraceptives 3.544304e-02 0.2010075631
86 medication <-> cigday_1 3.275316e-03 0.0345360471
87 medication <-> V8 2.084232e-03 0.0800493604
88 contraceptives <-> contraceptives 2.126582e-01 1.0000000000
89 contraceptives <-> cigday_1 4.892405e-02 0.4277382803
90 contraceptives <-> V8 2.688833e-03 0.0856272484
91 cigday_1 <-> cigday_1 6.151859e-02 1.0000000000
92 cigday_1 <-> V8 1.509276e-03 0.0893623867
93 V8 <-> V8 4.636832e-03 1.0000000000
94 group <-> group 1.000000e+00 1.0000000000
95 int group 0.000000e+00 0.0000000000
96 int Matsmk 1.262500e+00 2.8513745747
97 int Matagg 1.100000e+00 3.6436779343
98 int FamScore 2.250000e-01 0.6186398880
99 int EduPar 6.062500e-01 2.6138976225
100 int n_trauma 1.964286e-01 0.8668873691
101 int Age 5.621377e-01 2.5795724974
102 int int_dis 1.300000e+00 2.8190466136
103 int medication 1.175000e+00 3.0729848569
104 int contraceptives 1.300000e+00 2.8190466136
105 int cigday_1 1.243750e-01 0.5014526157
106 int V8 5.286908e-01 7.7640983340
fixed par
1 TRUE 0
2 FALSE 1
3 FALSE 2
4 FALSE 3
5 FALSE 4
6 FALSE 5
7 FALSE 6
8 FALSE 7
9 FALSE 8
10 FALSE 9
11 FALSE 10
12 FALSE 11
13 FALSE 12
14 FALSE 13
15 FALSE 14
16 FALSE 15
17 FALSE 16
18 FALSE 17
19 FALSE 18
20 FALSE 19
21 FALSE 20
22 FALSE 21
23 FALSE 22
24 FALSE 23
26 TRUE 0
27 TRUE 0
28 TRUE 0
29 TRUE 0
30 TRUE 0
31 TRUE 0
32 TRUE 0
33 TRUE 0
34 TRUE 0
35 TRUE 0
36 TRUE 0
37 TRUE 0
38 TRUE 0
39 TRUE 0
40 TRUE 0
41 TRUE 0
42 TRUE 0
43 TRUE 0
44 TRUE 0
45 TRUE 0
46 TRUE 0
47 TRUE 0
48 TRUE 0
49 TRUE 0
50 TRUE 0
51 TRUE 0
52 TRUE 0
53 TRUE 0
54 TRUE 0
55 TRUE 0
56 TRUE 0
57 TRUE 0
58 TRUE 0
59 TRUE 0
60 TRUE 0
61 TRUE 0
62 TRUE 0
63 TRUE 0
64 TRUE 0
65 TRUE 0
66 TRUE 0
67 TRUE 0
68 TRUE 0
69 TRUE 0
70 TRUE 0
71 TRUE 0
72 TRUE 0
73 TRUE 0
74 TRUE 0
75 TRUE 0
76 TRUE 0
77 TRUE 0
78 TRUE 0
79 TRUE 0
80 TRUE 0
81 TRUE 0
82 TRUE 0
83 TRUE 0
84 TRUE 0
85 TRUE 0
86 TRUE 0
87 TRUE 0
88 TRUE 0
89 TRUE 0
90 TRUE 0
91 TRUE 0
92 TRUE 0
93 TRUE 0
94 TRUE 0
95 TRUE 0
96 TRUE 0
97 TRUE 0
98 TRUE 0
99 TRUE 0
100 TRUE 0
101 TRUE 0
102 TRUE 0
103 TRUE 0
104 TRUE 0
105 TRUE 0
106 TRUE 0Warning in lav_data_full(data = data, group = group, cluster = cluster, :
lavaan WARNING: exogenous variable(s) declared as ordered in data: Matsmk Matagg
int_dis medication contraceptives
Warning in lav_data_full(data = data, group = group, cluster = cluster, : lavaan
WARNING: some estimated ov variances are negative############################
############################
Epi_M15
############################
############################
##Mediation Model ##
lavaan 0.6-7 ended normally after 107 iterations
Estimator DWLS
Optimization method NLMINB
Number of free parameters 23
Used Total
Number of observations 80 99
Model Test User Model:
Test statistic 72.096
Degrees of freedom 3
P-value (Chi-square) 0.000
Parameter Estimates:
Standard errors Standard
Information Expected
Information saturated (h1) model Unstructured
Regressions:
Estimate Std.Err z-value P(>|z|)
Epi ~
Matsmk (a) -0.055 0.057 -0.964 0.335
Matagg (b) 0.074 0.106 0.695 0.487
FamScore (c) 0.113 0.082 1.378 0.168
EduPar (d) 0.229 0.104 2.213 0.027
n_trauma (e) -0.062 0.089 -0.701 0.483
Age -0.088 0.121 -0.727 0.467
int_dis -0.065 0.047 -1.378 0.168
medication -0.024 0.056 -0.433 0.665
contrcptvs 0.032 0.055 0.579 0.562
cigday_1 -0.052 0.113 -0.458 0.647
V8 0.007 0.271 0.026 0.979
group ~
Matsmk (f) 0.193 1.158 0.167 0.867
Matagg (g) 1.614 2.501 0.645 0.519
FamScore (h) 0.202 2.105 0.096 0.923
EduPar (i) -3.176 2.248 -1.413 0.158
n_trauma (j) 2.329 1.439 1.618 0.106
Age -2.983 2.519 -1.184 0.236
int_dis 1.212 0.824 1.472 0.141
medication 1.137 0.868 1.309 0.191
contrcptvs 0.177 0.800 0.221 0.825
cigday_1 10.340 7.205 1.435 0.151
V8 13.279 16.898 0.786 0.432
Epi (z) -1.141 0.027 -41.523 0.000
Intercepts:
Estimate Std.Err z-value P(>|z|)
.Epi 0.000
.group 0.000
Thresholds:
Estimate Std.Err z-value P(>|z|)
group|t1 10.125
Variances:
Estimate Std.Err z-value P(>|z|)
.Epi 1.000
.group -0.302
Scales y*:
Estimate Std.Err z-value P(>|z|)
group 1.000
Defined Parameters:
Estimate Std.Err z-value P(>|z|)
directMatsmk 0.193 1.158 0.167 0.867
directMatagg 1.614 2.501 0.645 0.519
directFamScore 0.202 2.105 0.096 0.923
directEduPar -3.176 2.248 -1.413 0.158
directn_trauma 2.329 1.439 1.618 0.106
EpiMatsmk 0.063 0.065 0.963 0.335
EpiMatagg -0.084 0.121 -0.695 0.487
EpiFamScore -0.129 0.093 -1.377 0.169
EpiEduPar -0.261 0.118 -2.210 0.027
Epin_trauma 0.071 0.101 0.701 0.483
total 0.823 4.371 0.188 0.851
label lhs edge rhs est std group
1 int Epi 0.000000e+00 0.0000000000
2 a Matsmk ~> Epi -5.522110e-02 -0.0243855820
3 b Matagg ~> Epi 7.387035e-02 0.0222419975
4 c FamScore ~> Epi 1.127425e-01 0.0408962143
5 d EduPar ~> Epi 2.291129e-01 0.0529985053
6 e n_trauma ~> Epi -6.219036e-02 -0.0140545193
7 Age ~> Epi -8.796430e-02 -0.0191184353
8 int_dis ~> Epi -6.492649e-02 -0.0298616580
9 medication ~> Epi -2.404003e-02 -0.0091677648
10 contraceptives ~> Epi 3.188491e-02 0.0146648385
11 cigday_1 ~> Epi -5.184015e-02 -0.0128239070
12 V8 ~> Epi 7.148733e-03 0.0004855016
13 f Matsmk ~> group 1.932465e-01 0.0212707831
14 g Matagg ~> group 1.614209e+00 0.1211455206
15 h FamScore ~> group 2.022610e-01 0.0182873829
16 i EduPar ~> group -3.175795e+00 -0.1831093095
17 j n_trauma ~> group 2.329237e+00 0.1312050331
18 Age ~> group -2.982804e+00 -0.1615899741
19 int_dis ~> group 1.212276e+00 0.1389752499
20 medication ~> group 1.136593e+00 0.1080381857
21 contraceptives ~> group 1.766922e-01 0.0202559824
22 cigday_1 ~> group 1.034025e+01 0.6375719679
23 V8 ~> group 1.327938e+01 0.2247932544
24 z Epi ~> group -1.141079e+00 -0.2844197401
26 Epi <-> Epi 1.000000e+00 0.9947223019
27 group <-> group -3.020622e-01 -0.0186674925
28 Matsmk <-> Matsmk 1.960443e-01 1.0000000000
29 Matsmk <-> Matagg 4.936709e-02 0.3693241433
30 Matsmk <-> FamScore -9.177215e-03 -0.0569887592
31 Matsmk <-> EduPar -5.564346e-03 -0.0541843459
32 Matsmk <-> n_trauma 7.459313e-03 0.0743497863
33 Matsmk <-> Age -3.217300e-03 -0.0333441329
34 Matsmk <-> int_dis 2.151899e-02 0.1053910232
35 Matsmk <-> medication 4.113924e-03 0.0242997446
36 Matsmk <-> contraceptives 2.151899e-02 0.1053910232
37 Matsmk <-> cigday_1 1.693829e-02 0.1542372547
38 Matsmk <-> V8 2.928139e-03 0.0971189320
39 Matagg <-> Matagg 9.113924e-02 1.0000000000
40 Matagg <-> FamScore 3.417722e-02 0.3112715087
41 Matagg <-> EduPar -1.656118e-02 -0.2365241196
42 Matagg <-> n_trauma 7.233273e-03 0.1057402114
43 Matagg <-> Age 3.118694e-04 0.0047405101
44 Matagg <-> int_dis 3.291139e-02 0.2364027144
45 Matagg <-> medication 7.594937e-03 0.0657951695
46 Matagg <-> contraceptives 7.594937e-03 0.0545544726
47 Matagg <-> cigday_1 1.018987e-02 0.1360858260
48 Matagg <-> V8 8.272067e-04 0.0402393217
49 FamScore <-> FamScore 1.322785e-01 1.0000000000
50 FamScore <-> EduPar -2.948312e-02 -0.3495149022
51 FamScore <-> n_trauma 2.667269e-02 0.3236534989
52 FamScore <-> Age 3.636947e-03 0.0458878230
53 FamScore <-> int_dis 6.455696e-02 0.3849084009
54 FamScore <-> medication 4.430380e-03 0.0318580293
55 FamScore <-> contraceptives 5.822785e-02 0.3471722832
56 FamScore <-> cigday_1 4.381329e-02 0.4856887960
57 FamScore <-> V8 7.814844e-04 0.0315547719
58 EduPar <-> EduPar 5.379307e-02 1.0000000000
59 EduPar <-> n_trauma -8.024412e-03 -0.1526891136
60 EduPar <-> Age 2.762108e-03 0.0546490350
61 EduPar <-> int_dis -1.909283e-02 -0.1785114035
62 EduPar <-> medication 1.017932e-02 0.1147832062
63 EduPar <-> contraceptives -1.329114e-02 -0.1242676068
64 EduPar <-> cigday_1 -1.493803e-02 -0.2596730517
65 EduPar <-> V8 -8.860887e-06 -0.0005610523
66 n_trauma <-> n_trauma 5.134332e-02 1.0000000000
67 n_trauma <-> Age 1.582278e-03 0.0320439451
68 n_trauma <-> int_dis 4.159132e-02 0.3980335009
69 n_trauma <-> medication 1.763110e-02 0.2034979577
70 n_trauma <-> contraceptives 1.808318e-02 0.1730580439
71 n_trauma <-> cigday_1 2.128165e-02 0.3786692420
72 n_trauma <-> V8 -4.694340e-04 -0.0304243917
73 Age <-> Age 4.748866e-02 1.0000000000
74 Age <-> int_dis 8.090259e-03 0.0805056484
75 Age <-> medication -1.655660e-03 -0.0198700345
76 Age <-> contraceptives 3.524124e-02 0.3506833348
77 Age <-> cigday_1 8.542355e-03 0.1580445206
78 Age <-> V8 -1.333633e-03 -0.0898732659
79 int_dis <-> int_dis 2.126582e-01 1.0000000000
80 int_dis <-> medication 6.075949e-02 0.3445843938
81 int_dis <-> contraceptives 6.075949e-02 0.2857142857
82 int_dis <-> cigday_1 4.449367e-02 0.3890038953
83 int_dis <-> V8 5.645344e-03 0.1797788722
84 medication <-> medication 1.462025e-01 1.0000000000
85 medication <-> contraceptives 3.544304e-02 0.2010075631
86 medication <-> cigday_1 3.275316e-03 0.0345360471
87 medication <-> V8 2.084232e-03 0.0800493604
88 contraceptives <-> contraceptives 2.126582e-01 1.0000000000
89 contraceptives <-> cigday_1 4.892405e-02 0.4277382803
90 contraceptives <-> V8 2.688833e-03 0.0856272484
91 cigday_1 <-> cigday_1 6.151859e-02 1.0000000000
92 cigday_1 <-> V8 1.509276e-03 0.0893623867
93 V8 <-> V8 4.636832e-03 1.0000000000
94 group <-> group 1.000000e+00 1.0000000000
95 int group 0.000000e+00 0.0000000000
96 int Matsmk 1.262500e+00 2.8513745747
97 int Matagg 1.100000e+00 3.6436779343
98 int FamScore 2.250000e-01 0.6186398880
99 int EduPar 6.062500e-01 2.6138976225
100 int n_trauma 1.964286e-01 0.8668873691
101 int Age 5.621377e-01 2.5795724974
102 int int_dis 1.300000e+00 2.8190466136
103 int medication 1.175000e+00 3.0729848569
104 int contraceptives 1.300000e+00 2.8190466136
105 int cigday_1 1.243750e-01 0.5014526157
106 int V8 5.286908e-01 7.7640983340
fixed par
1 TRUE 0
2 FALSE 1
3 FALSE 2
4 FALSE 3
5 FALSE 4
6 FALSE 5
7 FALSE 6
8 FALSE 7
9 FALSE 8
10 FALSE 9
11 FALSE 10
12 FALSE 11
13 FALSE 12
14 FALSE 13
15 FALSE 14
16 FALSE 15
17 FALSE 16
18 FALSE 17
19 FALSE 18
20 FALSE 19
21 FALSE 20
22 FALSE 21
23 FALSE 22
24 FALSE 23
26 TRUE 0
27 TRUE 0
28 TRUE 0
29 TRUE 0
30 TRUE 0
31 TRUE 0
32 TRUE 0
33 TRUE 0
34 TRUE 0
35 TRUE 0
36 TRUE 0
37 TRUE 0
38 TRUE 0
39 TRUE 0
40 TRUE 0
41 TRUE 0
42 TRUE 0
43 TRUE 0
44 TRUE 0
45 TRUE 0
46 TRUE 0
47 TRUE 0
48 TRUE 0
49 TRUE 0
50 TRUE 0
51 TRUE 0
52 TRUE 0
53 TRUE 0
54 TRUE 0
55 TRUE 0
56 TRUE 0
57 TRUE 0
58 TRUE 0
59 TRUE 0
60 TRUE 0
61 TRUE 0
62 TRUE 0
63 TRUE 0
64 TRUE 0
65 TRUE 0
66 TRUE 0
67 TRUE 0
68 TRUE 0
69 TRUE 0
70 TRUE 0
71 TRUE 0
72 TRUE 0
73 TRUE 0
74 TRUE 0
75 TRUE 0
76 TRUE 0
77 TRUE 0
78 TRUE 0
79 TRUE 0
80 TRUE 0
81 TRUE 0
82 TRUE 0
83 TRUE 0
84 TRUE 0
85 TRUE 0
86 TRUE 0
87 TRUE 0
88 TRUE 0
89 TRUE 0
90 TRUE 0
91 TRUE 0
92 TRUE 0
93 TRUE 0
94 TRUE 0
95 TRUE 0
96 TRUE 0
97 TRUE 0
98 TRUE 0
99 TRUE 0
100 TRUE 0
101 TRUE 0
102 TRUE 0
103 TRUE 0
104 TRUE 0
105 TRUE 0
106 TRUE 0Warning in lav_data_full(data = data, group = group, cluster = cluster, :
lavaan WARNING: exogenous variable(s) declared as ordered in data: Matsmk Matagg
int_dis medication contraceptivesWarning in lav_samplestats_step2(UNI = FIT, wt = wt, ov.names = ov.names, :
lavaan WARNING: correlation between variables group and Epi is (nearly) 1.0Warning in lav_object_post_check(object): lavaan WARNING: some estimated ov
variances are negative############################
############################
Epi_M_all
############################
############################
##Mediation Model ##
lavaan 0.6-7 ended normally after 112 iterations
Estimator DWLS
Optimization method NLMINB
Number of free parameters 23
Used Total
Number of observations 80 99
Model Test User Model:
Test statistic 19.419
Degrees of freedom 3
P-value (Chi-square) 0.000
Parameter Estimates:
Standard errors Standard
Information Expected
Information saturated (h1) model Unstructured
Regressions:
Estimate Std.Err z-value P(>|z|)
Epi ~
Matsmk (a) 0.025 0.082 0.301 0.764
Matagg (b) 0.132 0.124 1.068 0.285
FamScore (c) -0.102 0.109 -0.934 0.350
EduPar (d) -0.281 0.169 -1.658 0.097
n_trauma (e) 0.074 0.126 0.589 0.556
Age 0.122 0.179 0.679 0.497
int_dis 0.085 0.074 1.148 0.251
medication 0.050 0.080 0.623 0.533
contrcptvs -0.074 0.084 -0.872 0.383
cigday_1 0.168 0.140 1.205 0.228
V8 0.285 1.060 0.268 0.788
group ~
Matsmk (f) 0.095 1.273 0.075 0.940
Matagg (g) 0.666 2.626 0.254 0.800
FamScore (h) 0.738 2.220 0.333 0.739
EduPar (i) -1.600 2.503 -0.639 0.523
n_trauma (j) 1.913 1.657 1.155 0.248
Age -3.678 2.775 -1.325 0.185
int_dis 0.728 0.955 0.763 0.446
medication 0.837 1.013 0.826 0.409
contrcptvs 0.622 0.970 0.641 0.521
cigday_1 9.298 7.262 1.280 0.200
V8 11.409 18.263 0.625 0.532
Epi (z) 6.542 0.104 63.023 0.000
Intercepts:
Estimate Std.Err z-value P(>|z|)
.Epi 0.000
.group 0.000
Thresholds:
Estimate Std.Err z-value P(>|z|)
group|t1 10.125
Variances:
Estimate Std.Err z-value P(>|z|)
.Epi 1.000
.group -41.801
Scales y*:
Estimate Std.Err z-value P(>|z|)
group 1.000
Defined Parameters:
Estimate Std.Err z-value P(>|z|)
directMatsmk 0.095 1.273 0.075 0.940
directMatagg 0.666 2.626 0.254 0.800
directFamScore 0.738 2.220 0.333 0.739
directEduPar -1.600 2.503 -0.639 0.523
directn_trauma 1.913 1.657 1.155 0.248
EpiMatsmk 0.161 0.535 0.301 0.764
EpiMatagg 0.864 0.809 1.068 0.286
EpiFamScore -0.665 0.712 -0.934 0.350
EpiEduPar -1.837 1.108 -1.657 0.097
Epin_trauma 0.487 0.827 0.589 0.556
total 0.823 4.371 0.188 0.851
label lhs edge rhs est std group
1 int Epi 0.000000e+00 0.0000000000
2 a Matsmk ~> Epi 2.458188e-02 0.0108076417
3 b Matagg ~> Epi 1.320289e-01 0.0395785961
4 c FamScore ~> Epi -1.016065e-01 -0.0366948417
5 d EduPar ~> Epi -2.807573e-01 -0.0646596116
6 e n_trauma ~> Epi 7.445977e-02 0.0167533797
7 Age ~> Epi 1.216017e-01 0.0263131775
8 int_dis ~> Epi 8.529333e-02 0.0390566463
9 medication ~> Epi 5.004593e-02 0.0190013736
10 contraceptives ~> Epi -7.362493e-02 -0.0337135711
11 cigday_1 ~> Epi 1.683723e-01 0.0414679492
12 V8 ~> Epi 2.846099e-01 0.0192441826
13 f Matsmk ~> group 9.543635e-02 0.0105047474
14 g Matagg ~> group 6.661429e-01 0.0499936421
15 h FamScore ~> group 7.383498e-01 0.0667577133
16 i EduPar ~> group -1.600452e+00 -0.0922785011
17 j n_trauma ~> group 1.913070e+00 0.1077624513
18 Age ~> group -3.677979e+00 -0.1992502669
19 int_dis ~> group 7.283543e-01 0.0834984647
20 medication ~> group 8.366138e-01 0.0795238067
21 contraceptives ~> group 6.219800e-01 0.0713037233
22 cigday_1 ~> group 9.297883e+00 0.5733000161
23 V8 ~> group 1.140922e+01 0.1931351063
24 z Epi ~> group 6.542238e+00 1.6378805576
26 Epi <-> Epi 1.000000e+00 0.9860014692
27 group <-> group -4.180088e+01 -2.5832993986
28 Matsmk <-> Matsmk 1.960443e-01 1.0000000000
29 Matsmk <-> Matagg 4.936709e-02 0.3693241433
30 Matsmk <-> FamScore -9.177215e-03 -0.0569887592
31 Matsmk <-> EduPar -5.564346e-03 -0.0541843459
32 Matsmk <-> n_trauma 7.459313e-03 0.0743497863
33 Matsmk <-> Age -3.217300e-03 -0.0333441329
34 Matsmk <-> int_dis 2.151899e-02 0.1053910232
35 Matsmk <-> medication 4.113924e-03 0.0242997446
36 Matsmk <-> contraceptives 2.151899e-02 0.1053910232
37 Matsmk <-> cigday_1 1.693829e-02 0.1542372547
38 Matsmk <-> V8 2.928139e-03 0.0971189320
39 Matagg <-> Matagg 9.113924e-02 1.0000000000
40 Matagg <-> FamScore 3.417722e-02 0.3112715087
41 Matagg <-> EduPar -1.656118e-02 -0.2365241196
42 Matagg <-> n_trauma 7.233273e-03 0.1057402114
43 Matagg <-> Age 3.118694e-04 0.0047405101
44 Matagg <-> int_dis 3.291139e-02 0.2364027144
45 Matagg <-> medication 7.594937e-03 0.0657951695
46 Matagg <-> contraceptives 7.594937e-03 0.0545544726
47 Matagg <-> cigday_1 1.018987e-02 0.1360858260
48 Matagg <-> V8 8.272067e-04 0.0402393217
49 FamScore <-> FamScore 1.322785e-01 1.0000000000
50 FamScore <-> EduPar -2.948312e-02 -0.3495149022
51 FamScore <-> n_trauma 2.667269e-02 0.3236534989
52 FamScore <-> Age 3.636947e-03 0.0458878230
53 FamScore <-> int_dis 6.455696e-02 0.3849084009
54 FamScore <-> medication 4.430380e-03 0.0318580293
55 FamScore <-> contraceptives 5.822785e-02 0.3471722832
56 FamScore <-> cigday_1 4.381329e-02 0.4856887960
57 FamScore <-> V8 7.814844e-04 0.0315547719
58 EduPar <-> EduPar 5.379307e-02 1.0000000000
59 EduPar <-> n_trauma -8.024412e-03 -0.1526891136
60 EduPar <-> Age 2.762108e-03 0.0546490350
61 EduPar <-> int_dis -1.909283e-02 -0.1785114035
62 EduPar <-> medication 1.017932e-02 0.1147832062
63 EduPar <-> contraceptives -1.329114e-02 -0.1242676068
64 EduPar <-> cigday_1 -1.493803e-02 -0.2596730517
65 EduPar <-> V8 -8.860887e-06 -0.0005610523
66 n_trauma <-> n_trauma 5.134332e-02 1.0000000000
67 n_trauma <-> Age 1.582278e-03 0.0320439451
68 n_trauma <-> int_dis 4.159132e-02 0.3980335009
69 n_trauma <-> medication 1.763110e-02 0.2034979577
70 n_trauma <-> contraceptives 1.808318e-02 0.1730580439
71 n_trauma <-> cigday_1 2.128165e-02 0.3786692420
72 n_trauma <-> V8 -4.694340e-04 -0.0304243917
73 Age <-> Age 4.748866e-02 1.0000000000
74 Age <-> int_dis 8.090259e-03 0.0805056484
75 Age <-> medication -1.655660e-03 -0.0198700345
76 Age <-> contraceptives 3.524124e-02 0.3506833348
77 Age <-> cigday_1 8.542355e-03 0.1580445206
78 Age <-> V8 -1.333633e-03 -0.0898732659
79 int_dis <-> int_dis 2.126582e-01 1.0000000000
80 int_dis <-> medication 6.075949e-02 0.3445843938
81 int_dis <-> contraceptives 6.075949e-02 0.2857142857
82 int_dis <-> cigday_1 4.449367e-02 0.3890038953
83 int_dis <-> V8 5.645344e-03 0.1797788722
84 medication <-> medication 1.462025e-01 1.0000000000
85 medication <-> contraceptives 3.544304e-02 0.2010075631
86 medication <-> cigday_1 3.275316e-03 0.0345360471
87 medication <-> V8 2.084232e-03 0.0800493604
88 contraceptives <-> contraceptives 2.126582e-01 1.0000000000
89 contraceptives <-> cigday_1 4.892405e-02 0.4277382803
90 contraceptives <-> V8 2.688833e-03 0.0856272484
91 cigday_1 <-> cigday_1 6.151859e-02 1.0000000000
92 cigday_1 <-> V8 1.509276e-03 0.0893623867
93 V8 <-> V8 4.636832e-03 1.0000000000
94 group <-> group 1.000000e+00 1.0000000000
95 int group 0.000000e+00 0.0000000000
96 int Matsmk 1.262500e+00 2.8513745747
97 int Matagg 1.100000e+00 3.6436779343
98 int FamScore 2.250000e-01 0.6186398880
99 int EduPar 6.062500e-01 2.6138976225
100 int n_trauma 1.964286e-01 0.8668873691
101 int Age 5.621377e-01 2.5795724974
102 int int_dis 1.300000e+00 2.8190466136
103 int medication 1.175000e+00 3.0729848569
104 int contraceptives 1.300000e+00 2.8190466136
105 int cigday_1 1.243750e-01 0.5014526157
106 int V8 5.286908e-01 7.7640983340
fixed par
1 TRUE 0
2 FALSE 1
3 FALSE 2
4 FALSE 3
5 FALSE 4
6 FALSE 5
7 FALSE 6
8 FALSE 7
9 FALSE 8
10 FALSE 9
11 FALSE 10
12 FALSE 11
13 FALSE 12
14 FALSE 13
15 FALSE 14
16 FALSE 15
17 FALSE 16
18 FALSE 17
19 FALSE 18
20 FALSE 19
21 FALSE 20
22 FALSE 21
23 FALSE 22
24 FALSE 23
26 TRUE 0
27 TRUE 0
28 TRUE 0
29 TRUE 0
30 TRUE 0
31 TRUE 0
32 TRUE 0
33 TRUE 0
34 TRUE 0
35 TRUE 0
36 TRUE 0
37 TRUE 0
38 TRUE 0
39 TRUE 0
40 TRUE 0
41 TRUE 0
42 TRUE 0
43 TRUE 0
44 TRUE 0
45 TRUE 0
46 TRUE 0
47 TRUE 0
48 TRUE 0
49 TRUE 0
50 TRUE 0
51 TRUE 0
52 TRUE 0
53 TRUE 0
54 TRUE 0
55 TRUE 0
56 TRUE 0
57 TRUE 0
58 TRUE 0
59 TRUE 0
60 TRUE 0
61 TRUE 0
62 TRUE 0
63 TRUE 0
64 TRUE 0
65 TRUE 0
66 TRUE 0
67 TRUE 0
68 TRUE 0
69 TRUE 0
70 TRUE 0
71 TRUE 0
72 TRUE 0
73 TRUE 0
74 TRUE 0
75 TRUE 0
76 TRUE 0
77 TRUE 0
78 TRUE 0
79 TRUE 0
80 TRUE 0
81 TRUE 0
82 TRUE 0
83 TRUE 0
84 TRUE 0
85 TRUE 0
86 TRUE 0
87 TRUE 0
88 TRUE 0
89 TRUE 0
90 TRUE 0
91 TRUE 0
92 TRUE 0
93 TRUE 0
94 TRUE 0
95 TRUE 0
96 TRUE 0
97 TRUE 0
98 TRUE 0
99 TRUE 0
100 TRUE 0
101 TRUE 0
102 TRUE 0
103 TRUE 0
104 TRUE 0
105 TRUE 0
106 TRUE 0rmd_paths <-paste0(tempfile(c(names(Netlist))),".Rmd")
names(rmd_paths) <- names(Netlist)
for (n in names(rmd_paths)) {
sink(file = rmd_paths[n])
cat(" \n",
"```{r, echo = FALSE}",
"Netlist[[n]]",
"```",
sep = " \n")
sink()
}Interactive SEM plots
Only direct effects with a significant standardized effect of p<0.05 are shown.
for (n in names(rmd_paths)) {
cat(knitr::knit_child(rmd_paths[[n]],
quiet= TRUE))
file.remove(rmd_paths[[n]])
}
sessionInfo()R version 4.0.3 (2020-10-10)
Platform: x86_64-w64-mingw32/x64 (64-bit)
Running under: Windows 10 x64 (build 19042)
Matrix products: default
Random number generation:
RNG: Mersenne-Twister
Normal: Inversion
Sample: Rounding
locale:
[1] LC_COLLATE=German_Germany.1252 LC_CTYPE=German_Germany.1252
[3] LC_MONETARY=German_Germany.1252 LC_NUMERIC=C
[5] LC_TIME=German_Germany.1252
attached base packages:
[1] grid parallel stats4 stats graphics grDevices utils
[8] datasets methods base
other attached packages:
[1] plyr_1.8.6 scales_1.1.1
[3] RCircos_1.2.1 compareGroups_4.4.6
[5] readxl_1.3.1 RRHO_1.28.0
[7] webshot_0.5.2 visNetwork_2.0.9
[9] org.Hs.eg.db_3.12.0 AnnotationDbi_1.52.0
[11] xlsx_0.6.5 gprofiler2_0.2.0
[13] BiocParallel_1.24.1 kableExtra_1.3.1
[15] glmpca_0.2.0 knitr_1.30
[17] DESeq2_1.30.0 SummarizedExperiment_1.20.0
[19] Biobase_2.50.0 MatrixGenerics_1.2.0
[21] matrixStats_0.57.0 GenomicRanges_1.42.0
[23] GenomeInfoDb_1.26.2 IRanges_2.24.1
[25] S4Vectors_0.28.1 BiocGenerics_0.36.0
[27] forcats_0.5.0 stringr_1.4.0
[29] dplyr_1.0.2 purrr_0.3.4
[31] readr_1.4.0 tidyr_1.1.2
[33] tibble_3.0.4 tidyverse_1.3.0
[35] semPlot_1.1.2 lavaan_0.6-7
[37] viridis_0.5.1 viridisLite_0.3.0
[39] WGCNA_1.69 fastcluster_1.1.25
[41] dynamicTreeCut_1.63-1 ggplot2_3.3.3
[43] gplots_3.1.1 corrplot_0.84
[45] RColorBrewer_1.1-2 workflowr_1.6.2
loaded via a namespace (and not attached):
[1] coda_0.19-4 bit64_4.0.5 DelayedArray_0.16.0
[4] data.table_1.13.6 rpart_4.1-15 RCurl_1.98-1.2
[7] doParallel_1.0.16 generics_0.1.0 preprocessCore_1.52.1
[10] callr_3.5.1 lambda.r_1.2.4 RSQLite_2.2.2
[13] mice_3.12.0 chron_2.3-56 bit_4.0.4
[16] xml2_1.3.2 lubridate_1.7.9.2 httpuv_1.5.5
[19] assertthat_0.2.1 d3Network_0.5.2.1 xfun_0.20
[22] hms_1.0.0 rJava_0.9-13 evaluate_0.14
[25] promises_1.1.1 fansi_0.4.1 caTools_1.18.1
[28] dbplyr_2.0.0 igraph_1.2.6 DBI_1.1.1
[31] geneplotter_1.68.0 tmvnsim_1.0-2 Rsolnp_1.16
[34] htmlwidgets_1.5.3 futile.logger_1.4.3 ellipsis_0.3.1
[37] crosstalk_1.1.1 backports_1.2.0 pbivnorm_0.6.0
[40] annotate_1.68.0 vctrs_0.3.6 abind_1.4-5
[43] cachem_1.0.1 withr_2.4.1 HardyWeinberg_1.7.1
[46] checkmate_2.0.0 fdrtool_1.2.16 mnormt_2.0.2
[49] cluster_2.1.0 mi_1.0 lazyeval_0.2.2
[52] crayon_1.3.4 genefilter_1.72.0 pkgconfig_2.0.3
[55] nlme_3.1-151 nnet_7.3-15 rlang_0.4.10
[58] lifecycle_0.2.0 kutils_1.70 modelr_0.1.8
[61] VennDiagram_1.6.20 cellranger_1.1.0 rprojroot_2.0.2
[64] flextable_0.6.2 Matrix_1.2-18 regsem_1.6.2
[67] carData_3.0-4 boot_1.3-26 reprex_1.0.0
[70] base64enc_0.1-3 processx_3.4.5 whisker_0.4
[73] png_0.1-7 rjson_0.2.20 bitops_1.0-6
[76] KernSmooth_2.23-18 blob_1.2.1 arm_1.11-2
[79] jpeg_0.1-8.1 rockchalk_1.8.144 memoise_2.0.0
[82] magrittr_2.0.1 zlibbioc_1.36.0 compiler_4.0.3
[85] lme4_1.1-26 cli_2.2.0 XVector_0.30.0
[88] pbapply_1.4-3 ps_1.5.0 htmlTable_2.1.0
[91] formatR_1.7 Formula_1.2-4 MASS_7.3-53
[94] tidyselect_1.1.0 stringi_1.5.3 lisrelToR_0.1.4
[97] sem_3.1-11 yaml_2.2.1 OpenMx_2.18.1
[100] locfit_1.5-9.4 latticeExtra_0.6-29 tools_4.0.3
[103] matrixcalc_1.0-3 rstudioapi_0.13 uuid_0.1-4
[106] foreach_1.5.1 foreign_0.8-81 git2r_0.28.0
[109] gridExtra_2.3 farver_2.0.3 BDgraph_2.63
[112] digest_0.6.27 shiny_1.6.0 Rcpp_1.0.5
[115] broom_0.7.3 later_1.1.0.1 writexl_1.3.1
[118] gdtools_0.2.3 httr_1.4.2 psych_2.0.12
[121] colorspace_2.0-0 rvest_0.3.6 XML_3.99-0.5
[124] fs_1.5.0 truncnorm_1.0-8 splines_4.0.3
[127] statmod_1.4.35 xlsxjars_0.6.1 systemfonts_0.3.2
[130] plotly_4.9.3 xtable_1.8-4 jsonlite_1.7.2
[133] nloptr_1.2.2.2 futile.options_1.0.1 corpcor_1.6.9
[136] glasso_1.11 R6_2.5.0 Hmisc_4.4-2
[139] mime_0.9 pillar_1.4.7 htmltools_0.5.1.1
[142] glue_1.4.2 fastmap_1.1.0 minqa_1.2.4
[145] codetools_0.2-18 lattice_0.20-41 huge_1.3.4.1
[148] gtools_3.8.2 officer_0.3.16 zip_2.1.1
[151] GO.db_3.12.1 openxlsx_4.2.3 survival_3.2-7
[154] rmarkdown_2.6 qgraph_1.6.5 munsell_0.5.0
[157] GenomeInfoDbData_1.2.4 iterators_1.0.13 impute_1.64.0
[160] haven_2.3.1 reshape2_1.4.4 gtable_0.3.0